To measure 3-year care costs of breast, prostate, colorectal and lung cancers disaggregated by site and clinical stage.
MethodA retrospective observational design was employed to investigate care costs of cases recorded in the Registry of the Basque Country between 2010 and 2015. Data gathered included TNM stage and demographic, clinical and resource use variables. Total costs per patient with stage IV disease were calculated by combining generalized linear models with parametric survival analysis. Unit costs were obtained from the analytical accounting system of the Basque Health Service.
ResultsThe sample comprised 23,782 cancer cases (7801 colorectal, 5530 breast, 4802 prostate and 5649 lung cancer). The mean 3-year costs per patient with stage I to III disease were €11,323, €13,727, €8,651 and €12,023 for colorectal, breast, prostate and lung cancer, respectively. The most important cost components were surgery and chemotherapy. Total survival-adjusted costs until death for patients with stage IV disease (€27,568, €26,296, €16,151 and €15,931 for breast, colorectal, lung and prostate cancer, respectively) were higher than the 3-year costs for those with earlier-stage disease.
ConclusionsThis study quantitatively shows the pattern of changes in the economic burden of cancer throughout its natural history and the great magnitude of this burden for the health system. The use of indicators based on real-world data from each regional health service would allow cancer care in each region to be tailored to local population needs.
Determinar el coste del tratamiento de los cánceres de mama, próstata, colorrectal y pulmón según la localización y el estadio clínico.
MétodoSe utilizó un diseño observacional retrospectivo con los casos del Registro de Euskadi entre 2010 y 2015. Los datos incluyeron el estadio TNM, variables demográficas y clínicas, y uso de recursos. Los costes totales por paciente en estadio IV se calcularon combinando modelos lineales generalizados con el análisis paramétrico de supervivencia. Los costes unitarios se obtuvieron del sistema de contabilidad analítica del Servicio Vasco de Salud.
ResultadosLa muestra estuvo compuesta por 23.782 casos (7801 colorrectal, 5530 de mama, 4802 de próstata y 5649 de pulmón). Los costes medios por paciente a 3 años en estadio I a III fueron 11.323 €, 13.727 €, 8651 € y 12.023 € para los cánceres colorrectal, de mama, de próstata y de pulmón, respectivamente. Los costes para el estadio IV (27.568 €, 26.296 €, 16.151 € y 15.931 € para los cánceres de mama, colorrectal, de pulmón y de próstata, respectivamente) fueron mayores que en los estadios iniciales.
ConclusionesEste estudio muestra cuantitativamente el cambio en la carga económica del cáncer a lo largo de su evolución y la gran carga que supone para el sistema de salud. El uso de datos del mundo real de cada servicio de salud permitiría adaptar la atención del cáncer a las necesidades de la población local.
Cancer-related costs in the European Union were estimated to be €126 billion in 2009, and of these, the direct healthcare costs amounted to €51 billion (40%). Nonetheless, the burden it represents varies substantially between countries, annual costs per patient ranging from €16 in Bulgaria to €184 in Luxembourg.1 The measurement of economic burden also contributes to our understanding of cancer not only as a matter of individual clinical care but also as a public health issue.1 The epidemiological scale of breast cancer (BC), prostate cancer (PC), colorectal cancer (CRC) and lung cancer (LC) is substantial. In the Basque population, they account for approximately half the incidence of cancer and high percentage of cancer deaths (43% in men and 34% in women).2 Moreover, the incidence of BC is growing in developed countries, it now being the most frequent cancer in women.3 PC constitutes the most common cancer in men.2 CRC is the most common type of cancer in the European Union,3 causing almost one in five cancer deaths.2 Finally, the LC is the second most common cancer among men and the third among women in Spain and the European Union,3 causing one in ten deaths from cancer.2
Cancer has a large economic impact not only because of its incidence but also because its diagnosis and treatment incur very high costs that vary over time.1,4 The use of healthcare resources is concentrated both in the initial period after the diagnosis of non-metastatic cancer and at the end of a patient's life if recurrence occurs. When this is the case, patients’ costs over time follow a characteristic U-shape.4 Further, secondary prevention based on screening and tertiary prevention involving new treatments are leading to changes in the economic and epidemiological burden. Notably, the introduction of screening programs has changed the incidence, TNM stage distribution and survival in both BC and CRC.5–7 Therefore, to evaluate cancer care policies, it is crucial to measure the epidemiological and economic burden adjusting for stage.8 Nonetheless, most studies analyze cancer costs disaggregated only by site and not by TNM stage.1
The literature includes cost studies for both BC9 and CRC.10 The latter has been shown to be the cancer with the second highest costs after LC in the USA.11 Furthermore, studies associated with clinical practice have been carried out for both LC12 and PC.13 To date, however, the studies carried out in Spain have sought to describe the economic consequences of applying the new clinical practice guidelines14 or have reviewed small samples from individual medical records.15,16 According to the review by Andrade et al.,17 cancer cost studies in Spain are scarce. Data are available, however, from cancer registries that include clinical stage and can be linked with variables from administrative and clinical databases in an automated way to carry out more extensive studies. Experts have recognized the importance of using such administrative databases as a source in health services research aimed to inform decision-making, since they are shaped by the organizational model and clinical practice applied in each country.18 Faced with the limited external validity of clinical trials, oncological clinical practice (generating real-world data) provides the ideal setting to measure the costs of caring for cancer patients.18,19 As with other diseases, the information recorded in the administrative and clinical databases of health systems allows us to analyze the profile of resources actually used in the treatment of cancer.
The objective of this study was to measure the costs disaggregated by clinical stage of the healthcare resources used from the diagnosis of the disease until 3 years in the treatment of BC, CRC, PC and LC, in a cohort of patients diagnosed between 2010 and 2015 treated within the Basque Health Service.
MethodDesignThis study employed a retrospective observational design to investigate the total number of patients diagnosed between 2010 and 2015 with BC, CRC, PC and LC included in the Osakidetza Hospital Cancer Registries (OHCRs). The OHCR databases collect data on patients with cancer treated in public hospitals in the Basque Country. Furthermore, OHCRs use the TNM Classification,20 unlike population registries that apply the Surveillance, Epidemiology, and End Results Program Summary Staging system. The analysis was carried out separately for each stage (I, II, III and IV) and site (colorectal, prostate, breast and lung). Follow-up was from the date of diagnosis until the date of death or, in living cases, for 3 years. The information collected included both demographic variables (age, sex, date of diagnosis and date of death) and clinical variables (stage and site). To assess hospital resource use by each patient (considering hospitalizations, complications, outpatient consultations, hospital-at-home services, chemotherapy and radiotherapy), data were obtained from the corporate database of the Basque Health Service to complement that from the OHCR. Hormone therapy costs were also retrieved from the pharmacy database. Unit costs were supplied by the Analytical Accounting Department of the Basque Health Service. The study protocol was approved by the Basque Country Clinical Research Ethics Committee (PI2016099).
Estimation of costsAs the analysis was performed from the perspective of the Basque Public Health Service, it was limited to healthcare costs. That is, we did not include costs related to the overall social perspective, such as those due to loss of productivity in patients and their caregivers, social costs or other intangible costs.21 The 3-year costs of care for stages I, II and III were grouped into two periods: initial (diagnosis and initial treatment) and follow-up. Diagnostic costs included contacts with outpatient consultations or hospitalizations in wards of the medical departments related to each site in the 2 months prior to the date of diagnosis. The medical departments considered for each site are listed in Table I of the online Appendix. Initial treatment costs were retrieved for the first year after diagnosis. The costs of follow-up included the use of resources such as hospitalization, outpatient consultations, diagnostic tests, chemotherapy or pharmacological treatments during the second and third year. In stage IV patients, all resources used in the treatment of the patient from diagnosis to death or the end of the 3-year period were considered.
The Analytical Accounting Service of the Basque Health Service provided the costs of hospital admissions and these were calculated using the diagnosis-related group system. The same source provided the unit cost of the outpatient consultations (first and successive), hospital at home services and visits to the emergency department. The total costs of chemotherapy sessions were obtained by summing the costs of the personnel and medical care of the day hospital, and the corresponding drugs and their preparation. The prices of drugs were taken from the Hospital Drugs Database of the Basque Health Service. The Medicines Database of the General Council of Pharmacy Colleges provided the pharmaceutical company's sales price of each drug administered through the out-of-hospital pharmacy (hormone therapy in BC and PC). Radiotherapy session unit costs were calculated based on activity, investments and personnel costs. The equipment depreciation time was set to 10 years with a useful life for the facilities of 30 years. The unit costs of the radiotherapy session included the costs of external radiation therapy, medical consultations, and physical radiation support. The unit costs considered in the analysis are shown in Tables II and III of the online Appendix.
Statistical analysisA descriptive analysis of the cohorts by site and stage of cancer detection was performed using absolute and relative frequencies in the case of categorical variables, and the mean and standard deviation in the case of continuous variables. The non-parametric Mann-Whitney test was used to compare the medians of the costs by stage.
As in metastatic cases the use of resources is maintained over time and depends on the duration of the follow-up, the total costs per patient were calculated with generalized linear models (GLMs), explaining the costs as a function of the different characteristics of the patients (sex, age, follow-up and death).22 The lifetime costs of stage IV patients were obtained by including patient mean survival time, calculated through parametric survival analysis, in the GLMs as a covariate. The survival function with the best fit based on the Akaike Information Criteria statistic was selected. The analyses were carried out using the statistical programming language R, version 3.6.1.
ResultsThe study analyzed 23,782 patients classified by stage and site. The largest group was that of cases of CRC (7801; 32.8%), followed by those with BC (5530; 23.3%), LC (5649; 23.8%) and PC (4802; 20.2%). A higher percentage of cases were in men than in women in the CRC and LC groups, while men accounted for just 1% of the sample with BC. The characteristics of the patients and their profile of resource use disaggregated by site and stage are shown in Table 1. Most of the cases were in individuals over 60 years of age, with the exception of the BC, where half of the patients were younger than that age. The stage distribution varied by site: while CRC, BC and PC were mostly diagnosed at stages I-III, more than half of patients with LC (55% of cases) had stage IV disease at diagnosis.
Characteristics and use of resources of cancer cases disaggregated by site and stage.
| Variable | Colorectal | Breast | Prostate | Lung |
|---|---|---|---|---|
| Total | 7,801 | 5,530 | 4,802 | 5,649 |
| Sex | ||||
| Male | 4,934 (63.25%) | 56 (1.01%) | 4,802 (100.00%) | 4,396 (77.82%) |
| Female | 2,867 (36.75%) | 5,474 (98.99%) | - | 1,253 (22.18%) |
| Age group, years | ||||
| <50 | 339 (4.35%) | 1,360 (24.59%) | 39 (0.81%) | 352 (6.23%) |
| 50 - <60 | 1,291 (16.55%) | 1,409 (25.48%) | 630 (13.12%) | 1,255 (22.22%) |
| 60 - <70 | 2,208 (28.30%) | 1,252 (22.64%) | 2,113 (44.00%) | 1,799 (31.85%) |
| 70 - <80 | 2,315 (29.68%) | 887 (16.04%) | 1,822 (37.94%) | 1,524 (26.98%) |
| 80 - <90 | 1,532 (19.64%) | 564 (10.20%) | 186 (3.87%) | 673 (11.91%) |
| ≥90 | 116 (1.49%) | 58 (1.05%) | 12 (0.25%) | 46 (0.81%) |
| Diagnosis year | ||||
| 2010 | 1,371 (17.57%) | 1,026 (18.55%) | 794 (16.53%) | 982 (17.38%) |
| 2011 | 1,563 (20.04%) | 1,067 (19.29%) | 917 (19.10%) | 1,082 (19.15%) |
| 2012 | 1,699 (21.78%) | 1,166 (21.08%) | 1,009 (21.01%) | 1,130 (20.00%) |
| 2013 | 1,624 (20.82%) | 1,167 (21.10%) | 993 (20.68%) | 1,231 (21.79%) |
| 2014 | 1,544 (19.79%) | 1,104 (19.96%) | 1,089 (22.68%) | 1,224 (21.67%) |
| Stage at diagnosis | ||||
| Stage I | 1,714 (21.97%) | 2,377 (42.98%) | 760 (15.83%) | 728 (12.89%) |
| Stage II | 2,106 (27.00%) | 2,007 (36.29%) | 2,613 (54.41%) | 441 (7.81%) |
| Stage III | 2,231 (28.60%) | 785 (14.20%) | 916 (19.08%) | 1,400 (24.78%) |
| Stage IV | 1,750 (22.43%) | 361 (6.53%) | 513 (10.68%) | 3,080 (54.52%) |
| Follow-up | ||||
| Stage I | 4.33 (CI: 3.31-5.61) | 4.9 (CI: 3.81-6.1) | 4.38 (CI: 3.39-5.53) | 3.49 (CI: 2.33-4.78) |
| Stage II | 4.41 (CI: 3.16-5.84) | 4.83 (CI: 3.61-6.21) | 4.98 (CI: 3.72-6.3) | 2.41 (CI: 1.01-4.39) |
| Stage III | 4.06 (CI: 2.74-5.58) | 4.41 (CI: 3.2-5.96) | 4.84 (CI: 3.66-5.99) | 1.10 (CI: 0.47-2.57) |
| Stage IV | 1.11 (CI: 0.33-2.61) | 2.53 (CI: 0.78-3.87) | 2.21 (CI: 0.81-3.52) | 0.40 (CI: 0.13-0.96) |
| Alive at end of follow-up | ||||
| Stage I | 1,532 (89.38%) | 2,327 (97.90%) | 722 (95.00%) | 512 (70.33%) |
| Stage II | 1,746 (82.91%) | 1,886 (93.97%) | 2,527 (96.71%) | 198 (44.90%) |
| Stage III | 1,665 (74.63%) | 665 (84.71%) | 879 (95.96%) | 330 (23.57%) |
| Stage IV | 396 (22.63%) | 160 (44.32%) | 207 (40.35%) | 177 (5.75%) |
| Surgery | ||||
| Stage I | 1,220 (71.18%) | 2,004 (84.31%) | 552 (72.63%) | 362 (49.73%) |
| Stage II | 1,486 (70.56%) | 1,697 (84.55%) | 1,861 (71.22%) | 221 (50.11%) |
| Stage III | 1,539 (68.98%) | 645 (82.17%) | 652 (71.18%) | 664 (47.43%) |
| Stage IV | 1,251 (71.49%) | 317 (87.81%) | 371 (72.32%) | 1,501 (48.73%) |
| Radiotherapy | ||||
| Stage I | 281 (16.39%) | 1,719 (72.32%) | 260 (34.21%) | 336 (46.15%) |
| Stage II | 382 (18.14%) | 1,425 (71.00%) | 920 (35.21%) | 197 (44.67%) |
| Stage III | 374 (16.76%) | 581 (74.01%) | 327 (35.70%) | 619 (44.21%) |
| Stage IV | 299 (17.09%) | 264 (73.13%) | 165 (32.16%) | 1,441 (46.79%) |
| Chemotherapy | ||||
| Stage I | 624 (36.41%) | 1,106 (46.53%) | 91 (11.97%) | 365 (50.14%) |
| Stage II | 760 (36.09%) | 913 (45.49%) | 310 (11.86%) | 215 (48.75%) |
| Stage III | 830 (37.20%) | 367 (46.75%) | 114 (12.45%) | 676 (48.29%) |
| Stage IV | 679 (38.80%) | 185 (51.25%) | 68 (13.26%) | 1,514 (49.16%) |
CI: confidence intervals.
Table 2 and Figure I of the online Appendix present the different diagnostic and treatment costs of stage I to III patients. The mean 3-year costs for these stages across the set of sites was €11,471, mean total costs for these early stages differing by site, from the lowest costs for PC (€8629) to the highest for BC (€13,727), LC and CRC having intermediate mean costs (€12,024 and €11,323 respectively). The largest cost component was surgery and followed by chemotherapy. Table 3 shows the costs associated with stage IV disease over a 3-year follow-up. When classified by site, the raw costs followed the same pattern: BC being the most expensive (€20,690), followed by CRC (€19,423), LC (€13,503) and PC (€10,427).
Mean 3-year costs disaggregated by diagnosis, treatment and follow-up and by site in patients with stage I to III cancer.
| Colorectal | Breast | Prostate | Lung | |
|---|---|---|---|---|
| Stage I | N=1,714 | N=2,377 | N=760 | N=728 |
| Initial costs | €7,754 | €10,519 | €6,284 | €9,814 |
| Diagnosis cost | €1,100 | €251 | €950 | €1,981 |
| Surgery cost | €4,961 | €4,094 | €3,859 | €5,860 |
| Radiotherapy cost | €457 | €2,330 | €1,262 | €1,203 |
| Chemotherapy cost | €1,236 | €3,844 | €214 | €771 |
| Follow-up costs | €2,688 | €3,045 | €1,774 | €2,469 |
| Hormonotherapy cost | - € | €691 | €110 | - € |
| Three-year total costs | €10,442 | €13,564 | €8,058 | €12,283 |
| Stage II | N=2,106 | N=2,007 | N=2,613 | N=441 |
|---|---|---|---|---|
| Initial costs | €8,494 | €10,515 | €6,999 | €10,172 |
| Diagnosis cost | €1,128 | €256 | €961 | €2,029 |
| Surgery cost | €5,549 | €4,204 | €4,436 | €6,260 |
| Radiotherapy cost | €534 | €2,310 | €1,349 | €1,170 |
| Chemotherapy cost | €1,283 | €3,746 | €253 | €713 |
| Follow-up costs | €3,159 | €3,140 | €1,715 | €2,543 |
| Hormonotherapy cost | - € | €625 | €92 | - € |
| Three-year total costs | €11,653 | €13,655 | €8,714 | €12,715 |
| Stage III | N=2,231 | N=785 | N=916 | N=1,400 |
|---|---|---|---|---|
| Initial costs | €8,260 | €11,087 | €6,841 | €9,841 |
| Diagnosis cost | €1,033 | €316 | €791 | €2,143 |
| Surgery cost | €5,409 | €4,324 | €4,469 | €5,602 |
| Radiotherapy cost | €463 | €2,382 | €1,335 | €1,089 |
| Chemotherapy cost | €1,355 | €4,065 | €245 | €1,008 |
| Follow-up costs | €3,428 | €3,315 | €2,121 | €1,830 |
| Hormonotherapy cost | - € | €613 | €360 | - € |
| Three-year total costs | €11,688 | €14,402 | €8,862 | €11,671 |
Mean 3-year costs of stage IV patients stratified by site and disaggregated by diagnosis and type of treatment.
| Colorectal | Breast | Prostate | Lung | |
|---|---|---|---|---|
| N=1,750 | N=361 | N=513 | N=3,080 | |
| Diagnosis cost | €1,215 | €739 | €1,557 | €2,779 |
| In-hospitalizations | €6,734 | €6,132 | €5,074 | €5,924 |
| Radiotherapy cost | €454 | €2,420 | €1,248 | €1,211 |
| Chemotherapy cost | €10,059 | €9,145 | €705 | €3,173 |
| Hormonotherapy cost | - | €923 | €771 | - |
| Follow-up consultations and emergency room | €961 | €1,331 | €1,072 | €416 |
| Three-year total costs | €19,423 | €20,690 | €10,427 | €13,503 |
The parameters of the GLMs explaining the total costs of stage IV disease by site are shown in Table 4. Follow-up was significant for all sites, except LC. In the case of PC, death at the end of the period was also a significant covariate. The other covariates did not explain a significant percentage of the variance in the costs of stage IV disease. The Gompertz function gave the best fit and was used to extrapolate survival curves for patients with stage IV disease (Fig. I of the online Appendix). The mean survival time varied considerably by site (Table 5), ranging from 1.04 years for men with LC to 5.22 years for men with PC. The model allowed the mean annual costs and mean total costs per patient to be calculated as a function of survival for the four sites (Table 5). The mean costs of care for a patient with stage IV disease from diagnosis to death considering their mean survival rates was €20,066 for all sites in men and €17,504 for the three sites affecting women.
General linear model parameters for total costs in stage IV patients disaggregated by site.
| Colorectal | Breast | Prostate | Lung | |||||
|---|---|---|---|---|---|---|---|---|
| aOR | p | aOR | p | aOR | p | aOR | p | |
| Follow up | 1.075 | 0.002 | 1.110 | 0.005 | 1.075 | 0.015 | 1.010 | 0.507 |
| Age | 0.999 | 0.420 | 1.000 | 0.969 | 0.999 | 0.544 | 1.000 | 0.599 |
| Alive (yes) | 0.972 | 0.631 | 0.908 | 0.260 | 0.849 | 0.013 | 0.964 | 0.495 |
| Sex (male) | 0.967 | 0.309 | 0.910 | 0.671 | - | - | 0.973 | 0.212 |
aOR: adjusted odds ratio.
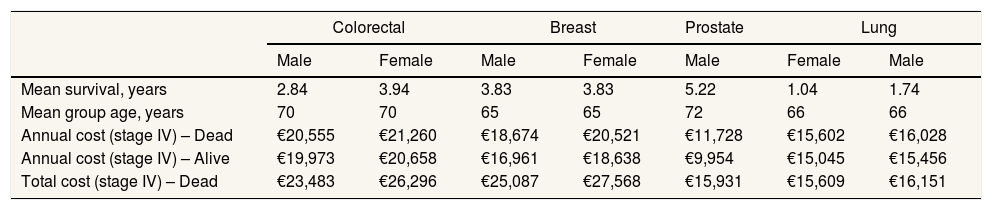
Costs associated with stage IV disease by site, sex, survival, and mean age of the group.
| Colorectal | Breast | Prostate | Lung | ||||
|---|---|---|---|---|---|---|---|
| Male | Female | Male | Female | Male | Female | Male | |
| Mean survival, years | 2.84 | 3.94 | 3.83 | 3.83 | 5.22 | 1.04 | 1.74 |
| Mean group age, years | 70 | 70 | 65 | 65 | 72 | 66 | 66 |
| Annual cost (stage IV) – Dead | €20,555 | €21,260 | €18,674 | €20,521 | €11,728 | €15,602 | €16,028 |
| Annual cost (stage IV) – Alive | €19,973 | €20,658 | €16,961 | €18,638 | €9,954 | €15,045 | €15,456 |
| Total cost (stage IV) – Dead | €23,483 | €26,296 | €25,087 | €27,568 | €15,931 | €15,609 | €16,151 |
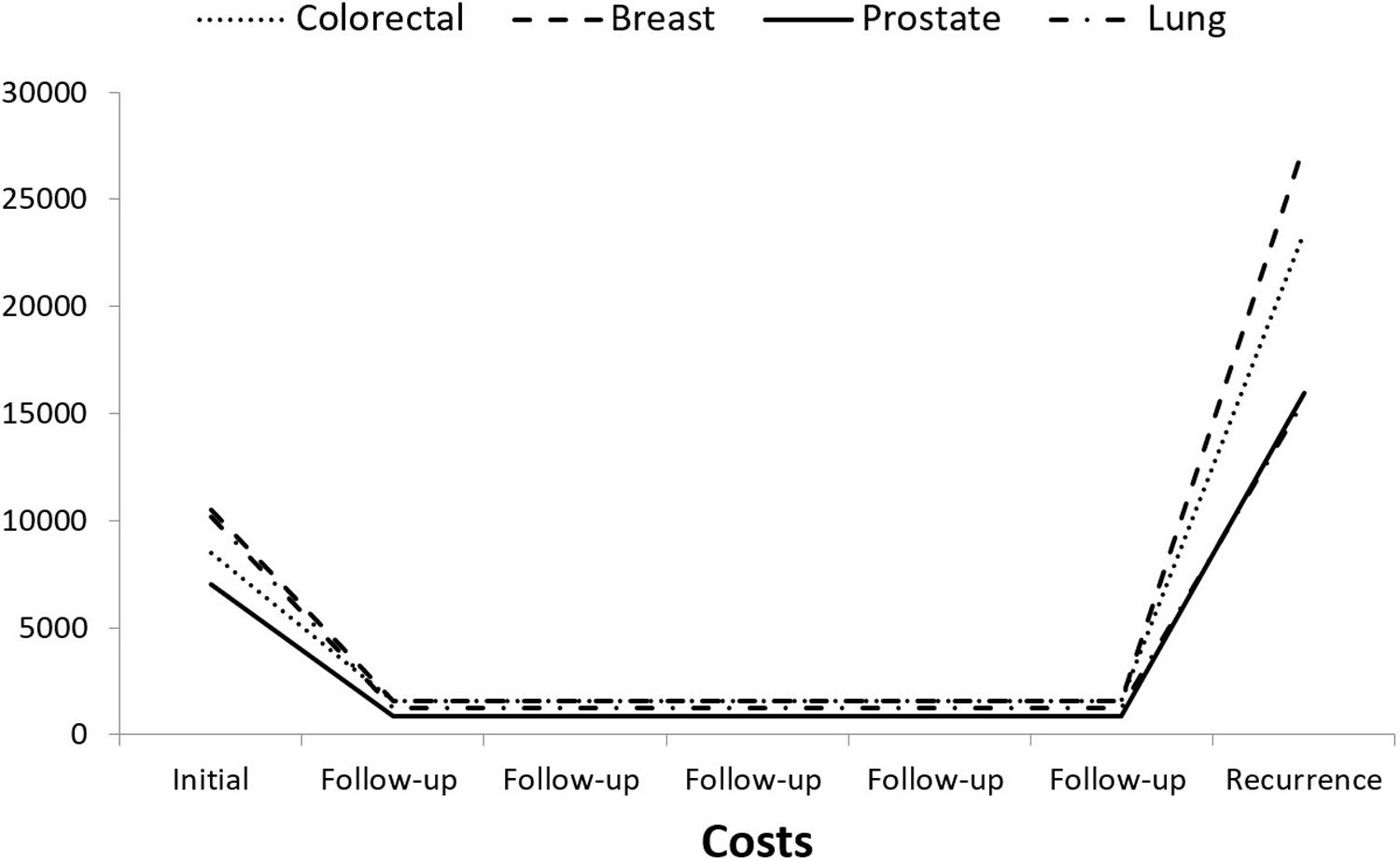
Figure 1 shows the U-shaped curve that represents the pattern of the costs of cancer care throughout its natural history when it ends with recurrence.4 The follow-up of 5 years was used to build the curve and is purely illustrative.
DiscussionThe main contribution of this study is to show the costs of healthcare for patients with cancer in the four most common sites disaggregated by stage and resource type in a database that recorded, for the first time in the Spanish cancer literature, the cases treated by a Regional Health Service for as long as 6 years. The breadth of the sample makes it possible to compare the magnitude and the components of the costs by stage and site. In stages I-III, the highest costs are those associated with BC due to the greater weight of chemotherapy and the costs are lowest for PC, with similar intermediate costs for CRC and LC. As could be expected, the use of resources varies depending on how advanced the disease is. For stage IV, the costs were higher than for the less advanced stages and CRC joins BC at the top of this list. The low survival in LC explains its costs being comparable to those of PC, which has lower annual costs but because of the longer survival requires longer treatment.
The two main cost components were surgery and chemotherapy, but resource use showed different profiles. While the costs of surgery at different sites were similar, the costs of chemotherapy differed markedly. The site with the highest chemotherapy costs was BC for stages I-III and CRC for stage IV. Despite being the main resource for treatment, surgery was used less frequently than observed in other studies.23 Further, in patients with PC, radiotherapy was less widely used than in other European countries,24 and consequently was associated with lower costs. The explanation for these differences is due in the case of surgery to the private provision of care in the USA and in the case of radiotherapy to the mixture of data sources used in the European study. In contrast, the rate of chemotherapy use is similar to that in other studies carried out in Spain, half (51%) of patients with LC receiving this type of treatment.15 On the other hand, comparison with studies in the USA indicated a lower rate of chemotherapy use in our setting.23 Follow-up costs differed between sites, hormone therapy constituting the bulk of follow-up treatment for both BC and PC.
In general, the mean total costs of stages I to III were similar to those previously published for Spain,10,15–17 but the present study provides more recent data. Similarly, the results achieved are comparable to those published in other European countries and lower than those in the USA and Canada.25,26 The average costs showed an increasing gradient with severity (stage) of the patient's disease, due to both procedures and complications of treatment, regardless of the site. Specifically, the mean total costs associated with CRC previously published in Spain range between €20,500 and €40,200,16 while in other countries the cost range is higher ($41,000-62,000).23,27 The mean total costs of BC (stages I to III: €13,727) were lower than those found in other studies in the Spanish population (€14,800-29,000),14,28 and significantly lower than those in the US population (stage I: $29,724; stage II: $39,322; stage III: $57,827).25 The costs related to PC (stages I to III: €8651) were higher than those indicated by other research in the Spanish population (€3338/year),24 but considerably lower than those found in the USA ($23,652).27 Finally, LC costs were less high than those shown by Corral et al.15 in Spain (€14,670), as well as those found in other countries ($17,800-39,900).23,26
Per-patient stage IV costs were always higher than the corresponding stage I-III costs and varied markedly between sites. On the other hand, differences were smaller when costs were adjusted for duration, the costs for the site with the lowest costs (PC) being closer to those for the other sites due to the longer survival. In the case of stage IV LC, the mean cost was similar to that reported in Spanish studies (€19,961)29 and the differences with respect to the total costs of the previous stages were lower. Further, in the cases of BC, CRC and LC, the adjusted costs of stage IV disease were similar to those shown by other studies.10,14,15,17
The methodology commonly used to analyze costs is usually the same for all stages without considering the specific characteristics of care for stage IV patients.15,16,27 Typically, stages I-III have a cost peak in the initial period followed by low follow-up cost. In contrast, stage IV patients’ costs can best be described through a continuous approach that reflects the sustained use of resources over time with an increase prior to death. The distribution of costs over time shown in the U-shaped curve (Fig. 1) justified our design. That is, we only assessed the first 3 years after diagnosis for stage I-III disease while stage IV costs were adjusted for duration. According to the GLMs, stage IV costs increased with follow-up but not proportionally.22 Our explanation is that longer survival in stage IV patients means they are more time in a good condition and, therefore, with a low resource use. Notably, follow-up was significant for BC, PC and CRC but not for LC due to the short survival. The duration and concentration of costs at certain time points are also the reasons for death only being associated with a significant increase in costs in PC.
These results serve to measure the burden of cancer and to inform both prevention and treatment policy decisions. Cancer treatment in the Basque Health Service hospitals is associated with a high use of resources but costs differ by stage and site. For these data to be relevant in the development of the Basque Oncological Plan, they must reflect the specific use of resources and costs incurred by the Basque Health Service. Moreover, the evaluation of the main cancer prevention policies in the Basque Country, namely, the CRC and BC screening programs, requires prior knowledge of the costs of each stage.5,30 The availability of corporate databases with information from the electronic medical record opens the possibility of analyzing all patients with a given type of cancer in a population. But the electronic medical record (real-world data) as a data source is not without problems.19 The use of real-world data in the decision-making process for its conversion into smart data requires the development of methodologies that resolve the lack of data on final health outcomes and the problems with data entry that occur in healthcare processes.18
The associated treatment cancer costs vary depending on patients’ stage, due to the treatment type change related to the disease severity. Also, treatment costs differ in terms of the cancer location. Thus, the costs of the most relevant cancers in terms of incidence can place a significant burden on the health system.
What does this study add to the literature?The costs of cancer treatment are very relevant with a much higher burden in the advanced cancer stages compared to initial stages. This burden is conditioned not only on the type of treatment administered, but also on a lower survival rate, which determines the costs, and the considered specific location.
David Cantarero.
Transparency declarationThe corresponding author on behalf of the other authors guarantee the accuracy, transparency and honesty of the data and information contained in the study, that no relevant information has been omitted and that all discrepancies between authors have been adequately resolved and described.
Authorship contributionsAll authors contributed to the study conception and design. Material preparation and data collection were performed by O. Ibarrondo, I. Larrañaga and M. Soto-Gordoa. Costs were calculated by G. Lizeaga and J.M. Martínez-Llorente. Statistical analysis was performed by O. Ibarrondo. Analysis of results was performed by I. Álvarez-López and O. Ibarrondo. The first draft of the manuscript was written by O. Ibarrondo and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
AcknowledgmentsThe authors want to acknowledge the rigorous work of Maria Luisa Iruretagoiena and the staff of the Osakidetza Hospital Cancer Registries, which has allowed this study. The authors also want to acknowledge Javier Mar and Arantzazu Arrospide for their help in methodological aspects. As well as Amaia Arriolabengoa for her extensive advice on hospital registration and their cost calculation. We also appreciate the contribution of all the volunteers who participated in the collection of funds from the EITB telethon. Lastly, we would like to acknowledge the help of Ideas Need Communicating Language Services in improving the use of English in the manuscript.
FundingThis work was funded by the BIO15 / CA / 013 / BD grant of the BIOEF cancer research grant round funded by the EITB Telethon.
Conflicts of interestsNone.