The 3rd International Nursing and Health Sciences Students and Health Care Professionals Conference (INHSP)
More infoBronchiectasis is a chronic infective and inflammatory respiratory disease that causes significant morbidity and mortality. Repeated respiratory infections may lead to infected bronchiectasis (IB) and acute exacerbations which often require hospital admission, increase risk of malnutrition and impact quality of life and eventually leads to death. Nutritional therapy is needed to modulate inflammation and enhance immunity to reduce severity of exacerbation, overcome malnutrition, as well as to decrease morbidity and mortality.
MethodsA 59-year-old female patient, diagnosed with IB. The patient had low oral intake due to productive cough and anorexia since 2 weeks before admission. Moreover, she had gradual shortness of breath that caused an impending respiratory failure during hospitalization, supported by continuous positive airway pressure (CPAP). Nutritional assessment was made based on Subjective Global Assessment (SGA) score C. Abnormal laboratory findings seen were increased in neutrophil-to-lymphocyte ratio (NLR) 9.3, moderate depletion of immune system with total lymphocyte count (TLC) 808.4/μl, hypoalbuminemia (3.2g/dl) and increased in liver enzymes: aspartate aminotransferase (AST) 206U/l, Alanine aminotransferase (ALT) 224U/l. Nutritional therapy was given gradually with target calorie 1400–1900kcal, protein 0.8–1.5g/kg IBW/day, carbohydrates 45–50%, and fat 33.3–43% through oral and parenteral nutrition. The patient was given supplementations such as vitamins (A, B complex, C, D), zinc, curcumin and snakehead fish extract high albumin content.
ResultAfter 14 days of treatment, significant clinical and metabolic improvement in NLR, TLC, plasma albumin, liver enzymes (AST/ALT), blood gas analysis, and functional capacity (handgrip strength) were found.
ConclusionAn adequate nutritional therapy with macro and micro-nutrients in IB patient can improve clinical outcome, nutritional status and quality of life.
Bronchiectasis non-cystic fibrosis (referred as bronchiectasis) is a progressive disease characterized by permanent bronchial dilatation, mucus retention and impaired clearance of cilia.1 The prevalence of bronchiectasis is not known with certainty and has historically been neglected. However, international data show an increase in the prevalence of bronchiectasis during the last few years in Europe and United States.2 The prevalence is higher in women and Asian populations.3 The widely known pathogenesis of bronchiectasis is based on the “Cole's Vicious Cycle Hypothesis” in which individuals at risk experience a strong inflammatory response to infection and lung tissue damage resulting in structural damage to the airway leading to mucus stasis, and support for chronic infection to continue as a vicious circle. The inflammatory response here involves neutrophils, lymphocytes, and macrophages.3
Patients with chronic airway diseases including bronchiectasis may experience nutritional deficiencies with the consequence of reduced muscle mass and impaired muscle function. Subsequent respiratory muscle dysfunction is associated with increased symptoms of breathlessness, poor response to exercise ventilation and exacerbations, and even lead to severe respiratory failure, and difficulty of weaning in mechanically ventilated patients. Providing nutritional therapy is very important and aims to increase energy intake, protein and amino acids especially branched chain amino acids (BCAA), foods rich in polyunsaturated fatty acids, vitamins and minerals in order to reduce local and systemic inflammation, replace muscle mass and treat nutrition disorders in patients with chronic airway diseases.4
MethodsA 59-year-old female patient was admitted to Infection Center of Wahidin Sudirohusodo Hospital in August 2019 with a complaint of shortness of breath since 5 days ago, she also has decreased oral intake experienced since 2 weeks ago due to decreased appetite and productive cough with very abundant mucus, yellowish colored. Nausea and vomiting existed 5 days ago. Unintentional weight loss has occurred since the last 2 weeks, approximately 4kg. She has a history of pulmonary tuberculosis with uncompleted anti-tuberculosis drug treatment, and recurrent cough and shortness of breath since 14 years ago. During hospitalization, the patient's clinical condition worsened due to impending respiratory failure. Her oxygen saturation was below <90% hence she had to receive oxygen support through continuous positive airway pressure (CPAP), and consequently became more difficult to eat and drink via oral because of chest tightness and dependent on oxygen support by CPAP mask and refused to insert a nasogastric tube.
At the time of consultation to clinical nutrition department, the patient was compos mentis with vital signs were: blood pressure 135/76mmHg, heart rate 90 beats per minute, respiratory rate 28 times per minute with oxygen supported by CPAP, oxygen saturation 98%, and body temperature 36.5°C. Physical examination showed base crackles on both of pulmonary fields and chest wall muscle retraction. There were muscle wasting at the lower and upper extremities. Abnormal laboratory findings seen were increased in neutrophil-to-lymphocyte ratio (NLR) 9.3, moderate depletion of immune system with total lymphocyte count (TLC) 808.4/μl, hypoalbuminemia (3.2g/dl) and elevated in liver transaminase: AST 206U/l, ALT 224U/l. Acid-resistant bacillus sputum test was negative. Gene expert: M. tuberculosis was undetected. Chest computed tomography (CT) scan result: active old pulmonary tuberculosis with infected bronchiectasis at the bottom on both lung fields, compensated pulmonary emphysema and cardiomegaly.
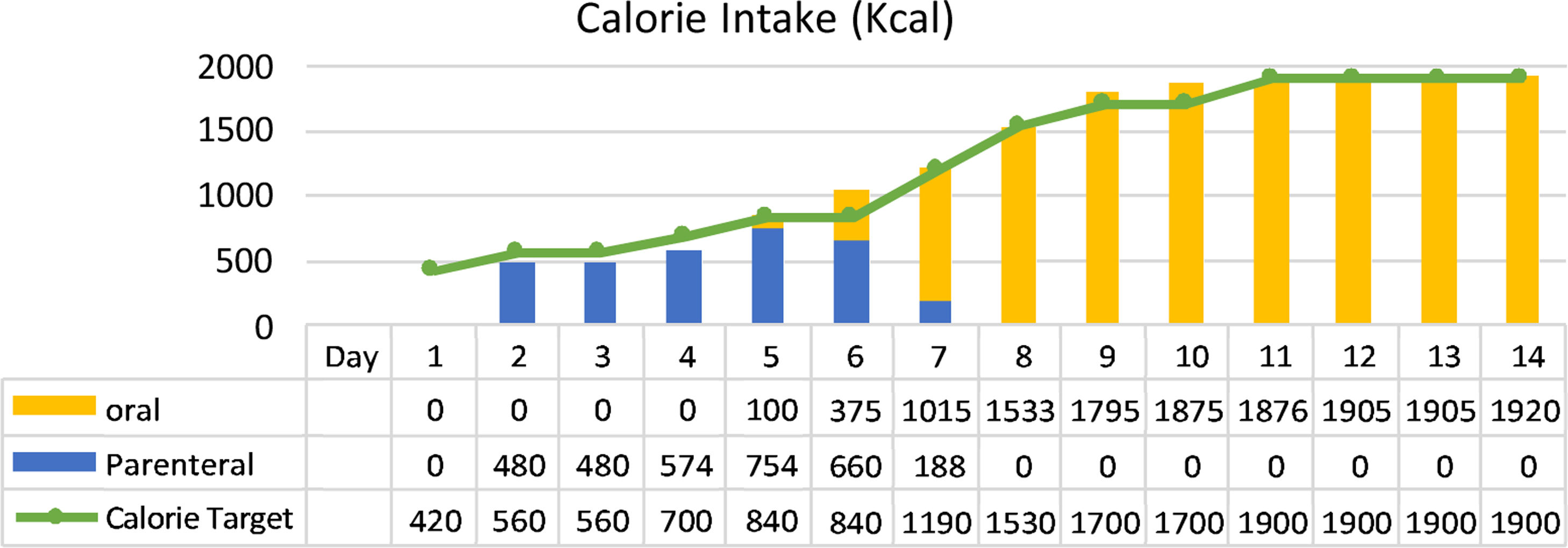
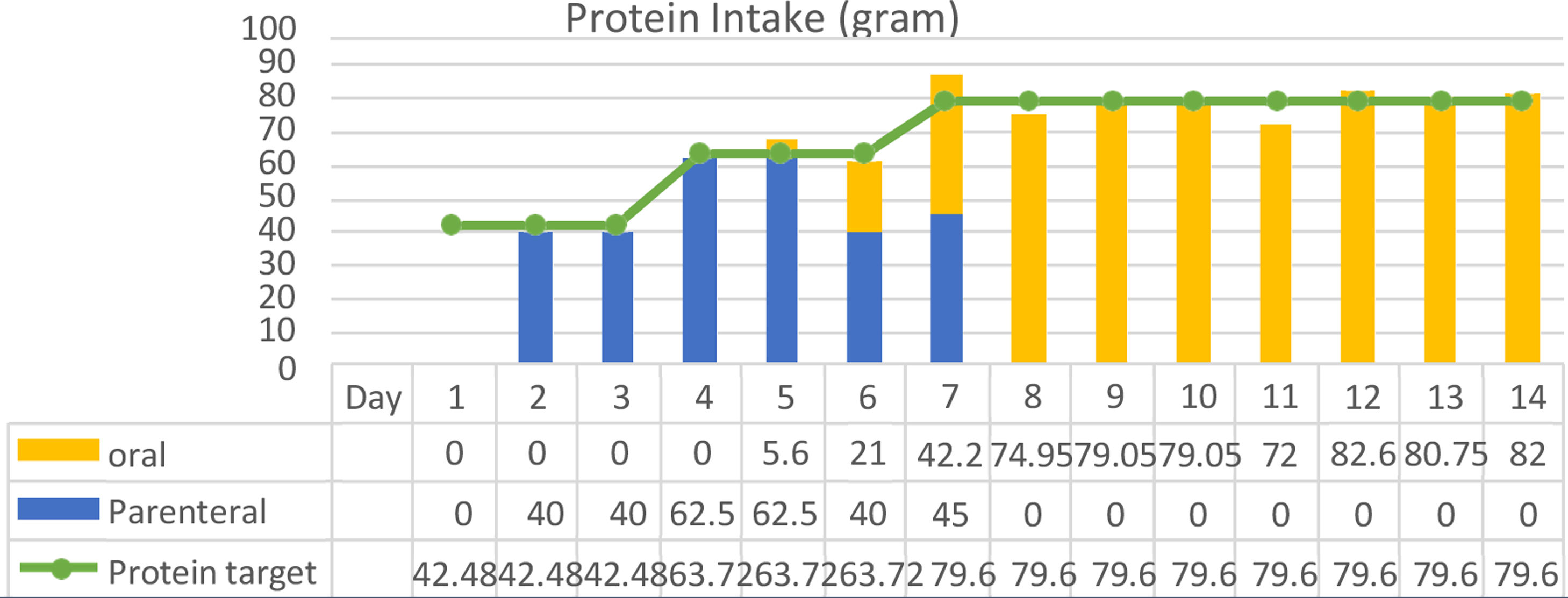
ResultThe clinical nutrition diagnosis for this patient was severe malnutrition (SGA score C). Nutritional therapy was given gradually with target calorie of 1400kcal calculated by Rule of thumb with protein 0.8–1.3g/kg IBW/day when the patient was in Intensive Care Unit (ICU) through parenteral and oral nutrition. After her condition was stable and transferred to general ward, target calorie increased to 1900kcal based on Harris Benedict equation. Macronutrient composition was protein 1.5g/kg IBW/day using high protein diet, carbohydrates 45–50%, and fat 33.3–38.3%. The patient was given supplementations such as vitamin A 6000IU, vitamin B complex, vitamin C 300mg, vitamin D 600IU, zinc 20mg, curcumin 1200mg and snakehead fish extract with high albumin content. The progression of nutritional support in calorie intake and protein can be achieved by the patient during treatment as shown in Figs. 1 and 2.
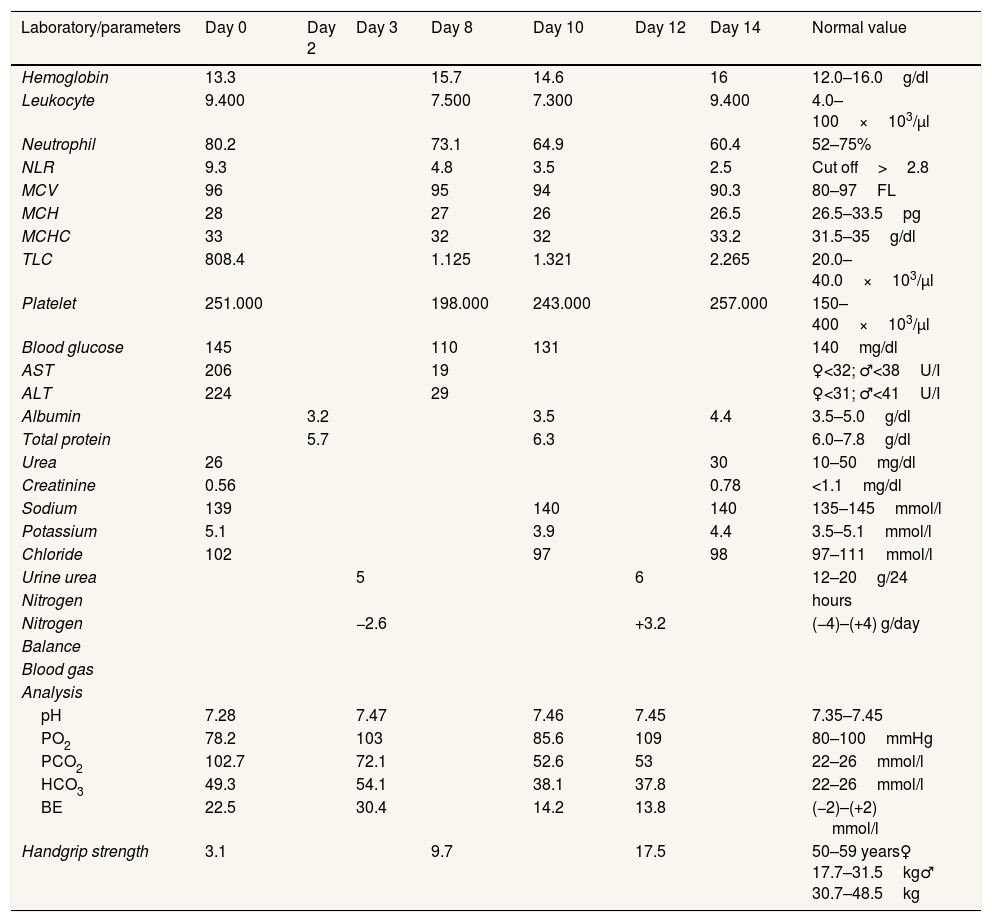
After 14 days of treatment, we found significant clinical and metabolic improvement, such as NLR 2.5, TLC 2265/μl, plasma albumin 4.4g/dl, nitrogen balance +3.2, and normal liver transaminase (AST 19, ALT 29U/l). In addition, there were improvements in arterial blood gas analysis test (increased oxygen pressure, decreased carbon dioxide pressure) due to resolved impending of respiratory failure problem and functional capacity (handgrip strength 3.1–17.5kg). All biochemical test results and parameters observed during hospitalization can be seen in Table 1.
All biochemical test results and parameters observed during hospitalization.
| Laboratory/parameters | Day 0 | Day 2 | Day 3 | Day 8 | Day 10 | Day 12 | Day 14 | Normal value |
|---|---|---|---|---|---|---|---|---|
| Hemoglobin | 13.3 | 15.7 | 14.6 | 16 | 12.0–16.0g/dl | |||
| Leukocyte | 9.400 | 7.500 | 7.300 | 9.400 | 4.0–100×103/μl | |||
| Neutrophil | 80.2 | 73.1 | 64.9 | 60.4 | 52–75% | |||
| NLR | 9.3 | 4.8 | 3.5 | 2.5 | Cut off>2.8 | |||
| MCV | 96 | 95 | 94 | 90.3 | 80–97FL | |||
| MCH | 28 | 27 | 26 | 26.5 | 26.5–33.5pg | |||
| MCHC | 33 | 32 | 32 | 33.2 | 31.5–35g/dl | |||
| TLC | 808.4 | 1.125 | 1.321 | 2.265 | 20.0–40.0×103/μl | |||
| Platelet | 251.000 | 198.000 | 243.000 | 257.000 | 150–400×103/μl | |||
| Blood glucose | 145 | 110 | 131 | 140mg/dl | ||||
| AST | 206 | 19 | ♀<32; ♂<38U/I | |||||
| ALT | 224 | 29 | ♀<31; ♂<41U/I | |||||
| Albumin | 3.2 | 3.5 | 4.4 | 3.5–5.0g/dl | ||||
| Total protein | 5.7 | 6.3 | 6.0–7.8g/dl | |||||
| Urea | 26 | 30 | 10–50mg/dl | |||||
| Creatinine | 0.56 | 0.78 | <1.1mg/dl | |||||
| Sodium | 139 | 140 | 140 | 135–145mmol/l | ||||
| Potassium | 5.1 | 3.9 | 4.4 | 3.5–5.1mmol/l | ||||
| Chloride | 102 | 97 | 98 | 97–111mmol/l | ||||
| Urine urea | 5 | 6 | 12–20g/24 | |||||
| Nitrogen | hours | |||||||
| Nitrogen | −2.6 | +3.2 | (−4)–(+4) g/day | |||||
| Balance | ||||||||
| Blood gas | ||||||||
| Analysis | ||||||||
| pH | 7.28 | 7.47 | 7.46 | 7.45 | 7.35–7.45 | |||
| PO2 | 78.2 | 103 | 85.6 | 109 | 80–100mmHg | |||
| PCO2 | 102.7 | 72.1 | 52.6 | 53 | 22–26mmol/l | |||
| HCO3 | 49.3 | 54.1 | 38.1 | 37.8 | 22–26mmol/l | |||
| BE | 22.5 | 30.4 | 14.2 | 13.8 | (−2)–(+2) mmol/l | |||
| Handgrip strength | 3.1 | 9.7 | 17.5 | 50–59 years♀ 17.7–31.5kg♂ 30.7–48.5kg | ||||
Abbreviations: NLR: neutrophil-to-lymphocyte count; MCV: mean corpuscular volume; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration; TLC: total lymphocyte count; AST: aspartate aminotransferase; ALT: alanine aminotransferase; PO2: oxygen pressure; PCO2: carbon monoxide pressure; HCO3: bicarbonate; BE: base excess.
Inflammation plays important role in IB and for modulating inflammation through nutrition therapy, we gave nutritional intake containing omega 3, BCAA and glutamine, as well as curcumin supplementation. Omega 3 provides a protective effect against pulmonary inflammation by increasing the resolution of inflammation through the production of specialized pro-resolving mediators such as resolvins, protectin and maresins.5 In a meta-analysis, omega-3 PUFAs in enteral diets might be associated with an improvement in early and late PaO2/FiO2 ratio, shortened length of stay in ICU and mechanical ventilation duration in patients with acute respiratory distress syndrome.6 BCAA can modulate inflammatory response of skeletal muscle through glutamine synthesis.7 In conditions of catabolic stress such as critical illness and respiratory disease, systemic glutamine depletion occurs because glutamine demand increases beyond the body's capacity to synthesize it.8 Yiming Xu and Ling Liu in their research concluded that the anti-inflammatory effects of curcumin has therapeutic potential for acute lung inflammatory disease.9 Previous study showed that NLR values were statistically greater in patients with bronchiectasis exacerbation compared to healthy controls.10 The higher NLR is associated with more inflammatory response.11 In our case, the patient had a decrease in NLR as an inflammatory biomarker after getting nutritional therapy.
In order to overcome the depletion of the immune system, we provided supplementation with vitamins A, C, D and mineral which is zinc. Shaker et al.12 demonstrated that providing zinc alone or simultaneous zinc and vitamin A supplementation in acute upper respiratory infections patients significantly improved the clinical outcome by enhancing the immune status. Alshami et al. (2018) in his editorial concluded that vitamin C plays role in improving of outcome in many respiratory diseases.13 Vitamin D deficiency tends to occur in bronchiectasis, and vitamin D supplementation can modulate immunity and affect the control of inflammation process.14 Bartley et al. (2018), revealed that vitamin D3 supplementation might increase their 25(OH)D levels and improved quality of life.15
The effect of pro-inflammatory cytokines causes changes in liver protein synthesis, specifically, increased in acute phase protein synthesis while the rate of albumin synthesis is down-regulated.16 Hypoalbuminemia was treated by administering a high protein diet containing BCAAs and snakehead fish extract with high albumin content. BCAAs especially leucine works by enhancing protein synthesis and attenuate proteolysis.7
Acute liver injury can result from hypoxic hepatitis.17 In our patient, impending respiratory failure caused the oxygen supply to the tissues consequently to be decreased, resulting acute liver injury due to hypoxia. When inflammation started to decline, the immune function was increased and respiratory problems were resolved, hence tissue perfusion was improved and seen in improvement of blood gas analysis. Moreover, liver enzyme levels returned to its normal values. In this case, we also found a progress in functional capacity, monitored by handgrip strength. Nutritional therapy with BCAAs has shown to increase muscle strength, fat free mass and improved quality of life in patients with chronic obstructive pulmonary disease.18
ConclusionAn adequate nutritional therapy with macro and micro-nutrients in IB patient could improve clinical outcome, nutritional status and quality of life.
Conflicts of interestThe authors declare no conflict of interest.
Peer-review under responsibility of the scientific committee of the 3rd International Nursing, Health Science Students & Health Care Professionals Conference. Full-text and the content of it is under responsibility of authors of the article.