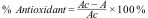The 3rd International Nursing and Health Sciences Students and Health Care Professionals Conference (INHSP)
More infoCocktail honey is derived from a mixture of honey (trigona sp.), bee bread, and homogeneous royal jelly. The material has a phenolic content rich in antioxidants that are beneficial for women's reproductive health, especially for pre-conception, because it can suppress the content of free radicals in the body. Antioxidants are useful to overcome oxidative damage due to free radicals in the body that prevent various diseases from increasing fertility during pre-conception.
MethodThis study used the DPPH (2,2-diphenyl-1-picrylhydrazyl) test method using UV–vis spectrophotometry to express the value of free radical reduction activity as IC50 (inhibitory concentration) values.
ResultsThe DPPH test on cocktail honey products obtained an average yield of 4577.7μg/mL, which was included in the product category was very weak in the antioxidant activity content.
ConclusionThe content contained in the honey cocktail contains weak bioactive content by assessing the antioxidant content using DPPH. The difference in the results of antioxidant activity tests using DPPH is caused by the test method and the conditions used in processing, homogeneous ingredients, solvent volume, extraction time, temperature, and pressure in product management.
Cocktail honey is a mixture of royal jelly, bee bread, and honey through mixing and laboratory test results.1 Royal jelly is a bee product other than honey which is produced from the hypopharyngeal glands of young bees containing polyphenols, several enzymes such as glucose oxidase, and superoxide dismutase, vitamins B1, B2, B3, and vitamin C, which act as antioxidants.2
Honey contains organic acids, amino acids, vitamins A, B complex, C, D, E, and K, electrolytes, elements such as copper, zinc, minerals with a pH between 3 and 4, enzymes, glucose, and fructose.3 Honey also plays an active role as an antioxidant.3 Honey also plays an active role as an antioxidant. With ingredients such as flavonoids, phenolic acids, enzymes, and vitamins.4
The Trigona genus bees produce trigona type honey.5 A study conducted by Nilawati et al. (2016) stated that trigona honey contains a high total phenolic amounting to 106mg/100g; vitamin E at 9.95μg/g; vitamin C 302.85μg/g; and quercetin of 58.8%.6 Trigono honey is a type of honey that comes from the forest, contains antioxidants that are high in flavonoids, vitamins, phenolic acids, and polyphenols.7
According to Oddo et al. (2008), research shows that the examination of the antioxidant activity of trigona honey using DPPH of 48.03ppm included in the category of strong antioxidants and flavonoid levels of 10.52mg.8
Bee bread is also a bee product from the fermentation of a mixture of pollen, nectar, and addition of bee saliva, which is inoculated by various bacteria and yeast, contains protein, lactic acid, which is a preservative,9 vitamins (C, B, K, P and E), minerals 3%, carbohydrates 24–35%, carotenoids, and polyphenols such as anthocyanins and flavonoids.10 As well as other active components such as the enzymes saccharase, amylase, phosphatase, a hormone that contains antioxidants.11
Various antioxidants have been found in plants with many phytochemicals with various bioactivities, including polyphenols, carotenoids, tocopherol, and ascorbic acid.12 Bee products contain antioxidants such as royal jelly, bee bread, and honey. The antioxidant activity of honey has been praised in previous studies and found that honey can suppress oxidation activity up to 50%, which is worth in the DPPH test 7.5–109mg/ml.13 Besides, other studies mention that bee bread contains phenolic compounds and flavonoids that play a role in antioxidant activity.14
This is in line with Rahma et al. (2014) research, stating that there are antioxidants in dorsata honey and trigona honey. The antioxidant action in honey can reduce or inhibit free radicals so that cell damage does not increase.15
This study was conducted to determine the antioxidant activity found in cocktail honey using the DPPH test method (2,2-diphenyl-1-picrylhydrazyl) using UV–vis spectrophotometry to know the value of free radical scavenging activity expressed by IC50 values (inhibitory concentration). It is expected to be one of the alternative therapies in treating oxidative stress in the body, especially in the reproductive problems of pre-conception women.
MethodsTest research from the honey cocktail sample, namely a mixture of 100g of royal jelly, 100g of bee bread, and 100g of Trigona honey, to see the antioxidant activity found in cocktail honey with the DPPH test.
Time and place of researchThe study was conducted in June 2020 in Makassar, South Sulawesi. Testing samples were done at two places, Mathematics and Natural Sciences Laboratory of the Faculty of Mathematics and Natural Sciences, Hasanuddin University, and the Biochemical Laboratory of Mathematics and Natural Sciences, Hasanuddin University, Makassar.
Tools and materialsThe tools used in this study are glassware (Pyrex), stopwatches, analytical scales, measuring flasks, pipettes, UV–vis spectrophotometers (Shimadzu type 2450). Materials used in this study include cocktail honey made from a mixture of 30ml honey:30ml bee bread:30ml royal jelly, a solution of methanol, distilled water, and 1,1-diphenyl-2-picrylhydrazyl (DPPH) pa (Sigma-Aldrich material code No. D9132-1G).
Research designThe making of cocktail honey is from a mixture of 30ml honey:30ml bee bread:30ml royal jelly. First, the prepared test solution is put into a test tube. Then, each tube was added with 1ml of 0.4mM DPPH solution, added 4ml of methanol to 5ml, and homogenized. The blank, test and comparative solutions were immediately incubated for 30min in a dark room of light. At the maximum wavelength of the Biochemical Laboratory of the Faculty of Mathematics and Natural Sciences, Hasanuddin University, the readings were 515nm using a UV–vis spectrophotometer. The uptake obtained was then recorded and calculated as a percentage of free radical activity inhibition.16 Thus, the value of the free radical damping activity will be known as stated by the IC50 (inhibitory concentration).
Research stagesThe raw material for making cocktail honey products is 30ml honey:30ml bee bread:30ml royal jelly obtained from the Faculty of Forestry, Hasanuddin University. The material is homogeneous until it merges. Cocktail honey products were tested for DPPH to determine the antioxidant activity by weighing 5mg DPPH dissolved with 20ml of absolute methanol in a flask.
Research parametersDPPH test (2,2-diphenyl-1-picrylhydrazyl) using UV–vis spectrophotometry due to finding out the antioxidant activity.3 A compound can be said to have antioxidant activity if the compound can donate its hydrogen atom to bind to DPPH to form a reduced DPPH characterized by looking at the change in color of each sample after incubation with DPPH. Increasingly the loss of purple or yellowing color.1 The determination of antioxidant activity is expressed in IC50 (μg/ml) as antioxidant capacity. The IC50 value is defined as the concentration of test compounds that can inhibit free radicals by as much as 50%. The smaller IC50 value, the higher the free radical reduction activity.3 The IC50 value category is powerful if the IC50 value <10μg/ml, strong if the IC50 value is between 10 and 50μg/ml, mild if the IC50 value is between 50 and 100μg/ml, weak if the IC50 value is between 100 and 250μg/ml and not active if IC50 is above 250μg/ml.1
Data processingData cannot be processed using statistical analysis because there is too little amount of data presented. The data is calculated using a formula to find the percentage value of the antioxidant activity, as well as the value of X or IC50 which is calculated using the line equation obtained from the % value of the antioxidant activity and the concentration value plotted on a graph, where the concentration value is on the X-axis and the % activity is on the Y-axis.
The concentration value comes from the value of the preliminary test on the sample carried out to find out one point or point desired or achieved. Absorbance value (A) with a maximum wavelength (λmax) of 515nm was obtained by testing using a spectrophotometer.
The effective concentration value is a number that shows the extract concentration (microgram/milliliter), which can inhibit 50% oxidation. The calculation of the effective concentration value or IC50 uses the following formula:
Information: Ac=absorbance value of control, A=the absorbance value of the sample.
ResultsAntioxidants are valuable compounds to overcome oxidative damage due to free radicals in the body to prevent various diseases.1 Although some research also states that flavonoid compounds can protect lipids from the oxidation of cell membranes, these compounds play a role in antioxidant activities that are beneficial to human health.17
In Tables 1–3 that have been plotted, the equation of the line is used to find the effective concentration of the honey cocktail to soak the DPPH free radicals or the IC50 value as shown in the following Figs. 1–3.
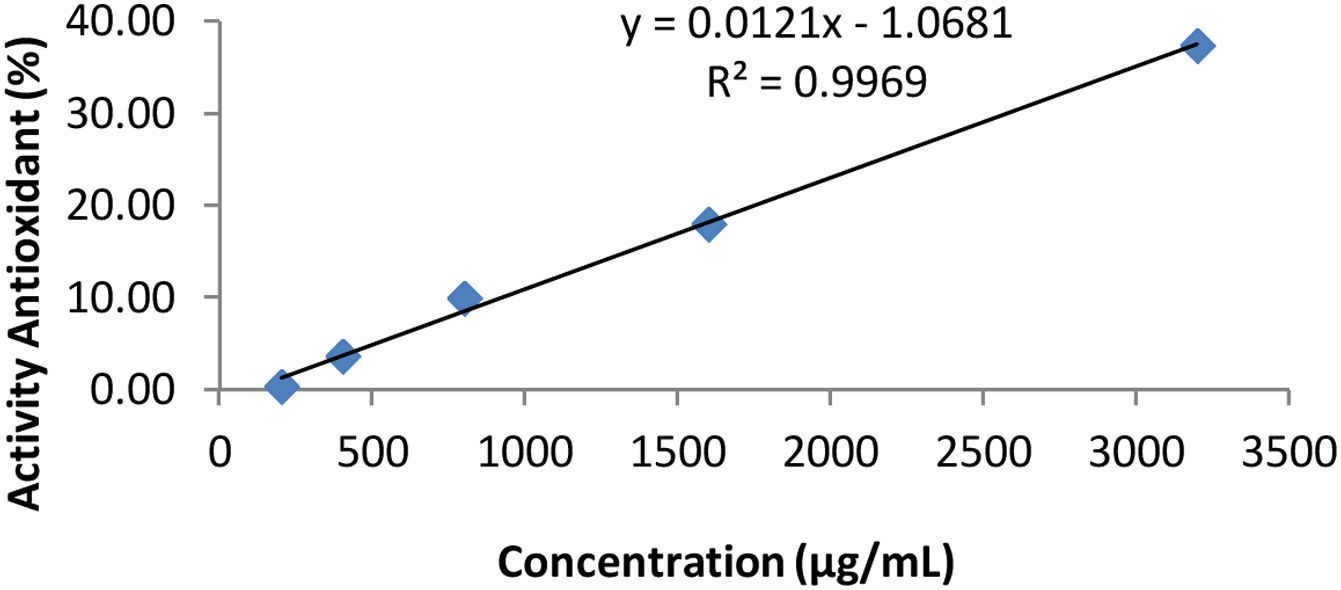
DPPH test results table on cocktail honey (Simplo).
| Concentration (μg/ml) | Absorbance (A) λ=515nm | Antioxidant activity (%) |
|---|---|---|
| 200 | 0.431 | 0.46 |
| 400 | 0.417 | 3.70 |
| 800 | 0.390 | 9.93 |
| 1600 | 0.355 | 18.01 |
| 3200 | 0.271 | 37.41 |
| Control | 0.433 |
Description: The data in Table 1 is regressed with variations in concentration as the X value and % antioxidant activity as the Y value according to the variation in the test method.
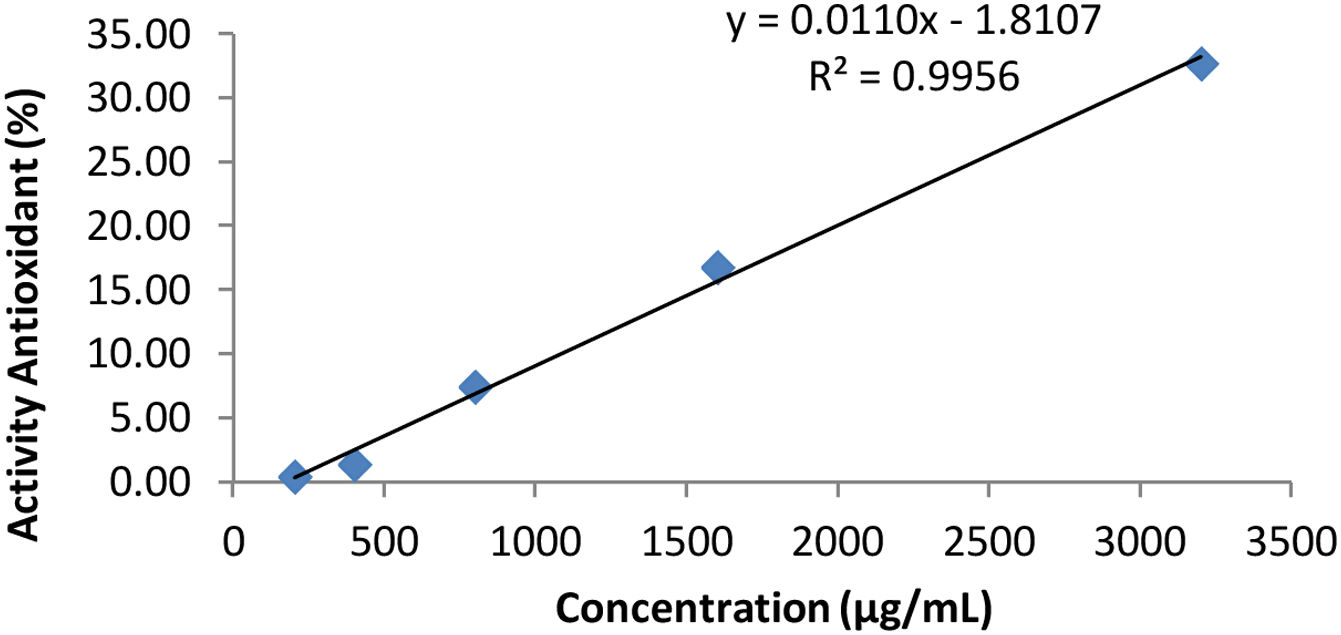
DPPH test results table on cocktail honey (Duplo).
| Concentration (μg/ml) | Absorbance (A) λ=515nm | Antioxidant activity (%) |
|---|---|---|
| 200 | 0.426 | 0.47 |
| 400 | 0.422 | 1.40 |
| 800 | 0.396 | 7.48 |
| 1600 | 0.356 | 16.82 |
| 3200 | 0.288 | 32.71 |
| Control | 0.428 |
Description: The data in Table 2 is regressed with variations in concentration as the X value and % antioxidant activity as the Y value according to the variation in the test method.
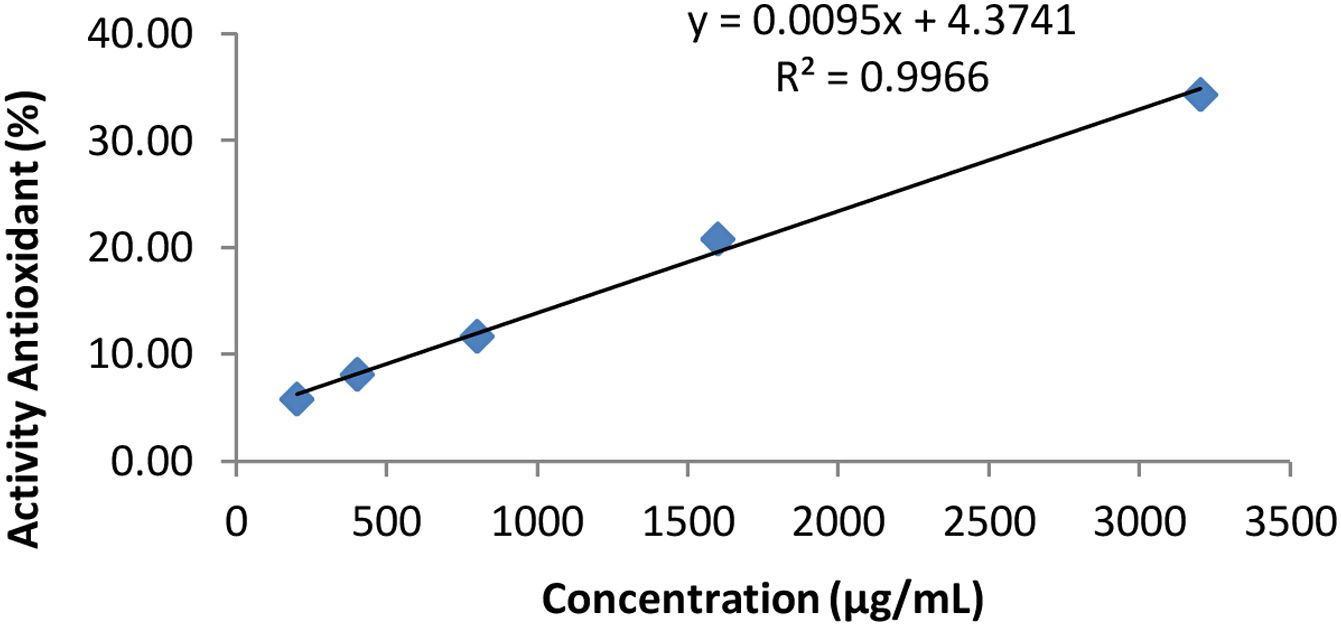
DPPH test results table on cocktail honey (Triplo).
| Concentration (μg/ml) | Absorbance (A) λ=515nm | Antioxidant activity (%) |
|---|---|---|
| 200 | 0.416 | 5.88 |
| 400 | 0.406 | 8.14 |
| 800 | 0.390 | 11.76 |
| 1600 | 0.350 | 20.81 |
| 3200 | 0.290 | 34.39 |
| Control | 0.442 |
Description: The data in Table 3 is regressed with variations in concentration as the X value and % antioxidant activity as the Y value according to the variation in the test method.
The curve of Table 1.
The curve of Table 2.
The curve of Table 3.
Based on Table 4, the IC50 value is the effective concentration of the extract needed to immerse 50% of the activity of the total DPPH, so that the IC50 value is substituted for the value. After substituting the value of 50 for the y value, the x value is obtained as the IC50 value. Based on the linear regression equation y=bx+a, the IC50 value for the honey cocktail product is obtained at simplo 4220.5041μg/ml, duplo of 4710.0636μg/ml, and triplo of 4802.7263μg/ml.
DiscussionThe IC50 simplo, duplo, and triplo values show that the IC50 value is >250μg/ml so that the honey cocktail contains very weak antioxidants (IC50 value >250). According to the parameters, The IC50 value category is very strong if the IC50 value <10μg/ml, strong if the IC50 value is between 10 and 50μg/ml, mild if the IC50 value is between 50 and 100μg/ml, weak if the IC50 value is between 100 and 250μg/ml and not active if IC50 is above 250μg/ml.1
The study conducted by Handayani (2018) stated that methanol extract gave positive results on tannins, flavonoids, saponins, alkaloids, and steroids with DPPH test IC50 values of 683.153μg/ml. At the same time, DCM extract gave positive results of steroids, alkaloids, and tannins with IC50 values of 701.743μg/ml. Thus, positive n-hexane extract containing alkaloids and tannins IC50 values 1709.536μg/ml, alkaloid positive water extracts, steroids, tannins, and saponins with IC50 values of 1698.345μg/ml. Positive honey samples contain all aspects tested with an IC50 value of 2826.471μg/ml, so it can be concluded that honey samples and each extract have a very weak antioxidant ability.7
Several factors can influence antioxidant activity, namely the differences in the types of honey-producing bees, the geographical conditions of the plant sources used by the bees.18 Flavonoids are one component of phenolic compounds which are natural antioxidants derived from plants. Plants that live in the grazing location will affect the content of flavonoids, which are natural antioxidants in the product.19
The phenolic content also depends on the location and geographic location in beekeeping, which has different geographies and different available plant sources so that the phenolic content also varies.20 The grazing area with a few plant species will affect the amount of flavonoid content in the product material because the limited number of plants in the grazing area can make it difficult for the bees to search for food due to the different bees flying range. For example, the genus Trigona sp. has a flying range of only 500m radii so that the bees incentivize in the hive area.19
ConclusionThe results showed that the content contained in the honey cocktail contains weak bioactive content by assessing the antioxidant content using DPPH. The phenolic content can cause this also depends on the location, geographic location of grazing, which has different geographical and different available plant sources so that the phenolic content also varies.20
The difference in the results of antioxidant activity tests using DPPH is caused by the test method and the conditions used in processing, homogeneous ingredients, solvent volume, extraction time, temperature, and pressure in product management. The extraction method process can also influence antioxidant activity, and the conditions used when making products.21
FundingThe author received no financial support for the research, authorship, and publication of this article.
Conflict of interestThe authors declare no conflict of interest.
The author is grateful to the publication Unit and Data Analysis of graduate School for their assistance and helpful comments, suggestions, and English language revision.
Peer-review under responsibility of the scientific committee of the 3rd International Nursing, Health Science Students & Health Care Professionals Conference. Full-text and the content of it is under responsibility of authors of the article.