In this study we conducted an economic evaluation of a surveillance programme to prevent hip dislocation in children with cerebral palsy.
MethodWe developed a model that compared costs and health outcomes of children with cerebral palsy with and without a surveillance programme. Information from a number of sources was combined into a decision analytical model, primarily based on data from a comparative study with a 20-year follow-up. Effectiveness was measured using Quality-Adjusted Life Years (QALYs). The analysis took the perspective of the Spanish National Health Service. We undertook extensive sensitivity analyses including a probabilistic sensitivity analysis.
ResultsThe surveillance programme led to higher QALYs and higher health care costs, with an estimated incremental cost per QALY gained of 12,282€. The results were robust to model assumptions. The probability that the programme was cost-effective was estimated to be over 80% at the threshold of 25.000€/QALY recommended in Spain.
ConclusionThis study indicates that surveillance programmes to prevent hip dislocation in children with cerebral palsy are likely to be cost-effective.
En este estudio se realiza una evaluación económica de un programa de vigilancia para prevenir la luxación de cadera en niños y niñas con parálisis cerebral.
MétodoSe desarrolló un modelo que comparó los costes y los resultados en salud de niños y niñas con parálisis cerebral incluidas y no incluidas en un programa de vigilancia. Se combinó la información proveniente de diversas fuentes en un modelo analítico de decisión, principalmente basado en datos de un estudio comparativo con 20 años de seguimiento. La efectividad se midió empleando los años de vida ajustados por calidad (AVAC). El análisis tomó la perspectiva del Sistema Nacional de Salud de España. Se realizó un extenso análisis de sensibilidad, incluyendo un análisis de sensibilidad probabilístico.
ResultadosEl programa de vigilancia estuvo asociado a más AVAC y mayores costes sanitarios, con un coste incremental por AVAC ganado estimado en 12.282 €. Los resultados fueron robustos a los supuestos del modelo. La probabilidad de que el programa fuera coste-efectivo se estimó en un valor por encima del 80% para el umbral de 25.000 € por AVAC recomendado en España.
ConclusiónEste estudio indica que es probable que los programas de vigilancia para prevenir la luxación de cadera en niños y niñas con parálisis cerebral sean coste-efectivos.
Cerebral palsy has an incidence of approximately two per 1000 live births and it is considered the most common cause of physical disability in children in developed countries.1 A common but often preventable complication in children with cerebral palsy is the dislocation of the hip, usually attributed to spasticity and contracture of the hip adductors and flexors as well as the medial hamstrings.2 Between 15-20% of children with cerebral palsy develop this condition.3
At first, children with cerebral palsy might experience asymptomatic subluxation or displacement of the hip that can progress into painful dislocation, contributing to difficulties with sitting, standing, walking, dressing, and perineal hygiene.4 In most cases children with identified displacement will need surgery to prevent dislocation.5 Treatments for hip displacement in children with cerebral palsy are less invasive and more successful in hips with less hip degenerative change and less displacement.6 Due to the silent nature of early stages of the development of hip displacement, screening or surveillance programs may permit early detection and treatment.
Surveillance programs involve the monitoring of children with cerebral palsy until they reach skeletal maturity based on standardized clinical evaluations and radiological examinations. Hip displacement is often evaluated using the Reimer index or migration percentage (MP)7, with most authors classifying hips with a MP>30% as displaced, and hips with an MP>90% to 100% as dislocated.2 The potential of surveillance to reduce hip dislocation depends on the appropriate planning of early treatment once displacement is detected. Surgical treatments to prevent dislocation include adductor–psoas tenotomy and varus osteotomy of the proximal femur. Salvage surgery (e.g. femoral head resection) is usually performed, if the child is fit to undergo surgery, when the hip has reached dislocation.8
Some countries and regions have established surveillance programs, achieving a reduction in the rate of hip dislocation.3,9–14 Well-applied surveillance programs have therefore been considered effective and practical.6 However, no previous study has provided evidence on the cost-effectiveness of this intervention.
The aim of this study is to determine the cost-effectiveness of a surveillance program to prevent dislocation of the hip in children with cerebral palsy in Spain.
MethodModel overviewIn this analysis we compared the costs and Quality-Adjusted Life Years (QALYs) of children with cerebral palsy with and without a surveillance program to prevent hip dislocation. The perspective was of the Spanish National Health System,15 with a time horizon of 18 years, coinciding approximately with the follow-up duration of available data. We applied a 3% discount rate to future costs and QALYs.15,16 The paper follows the CHEERS Statement for economic evaluations.17
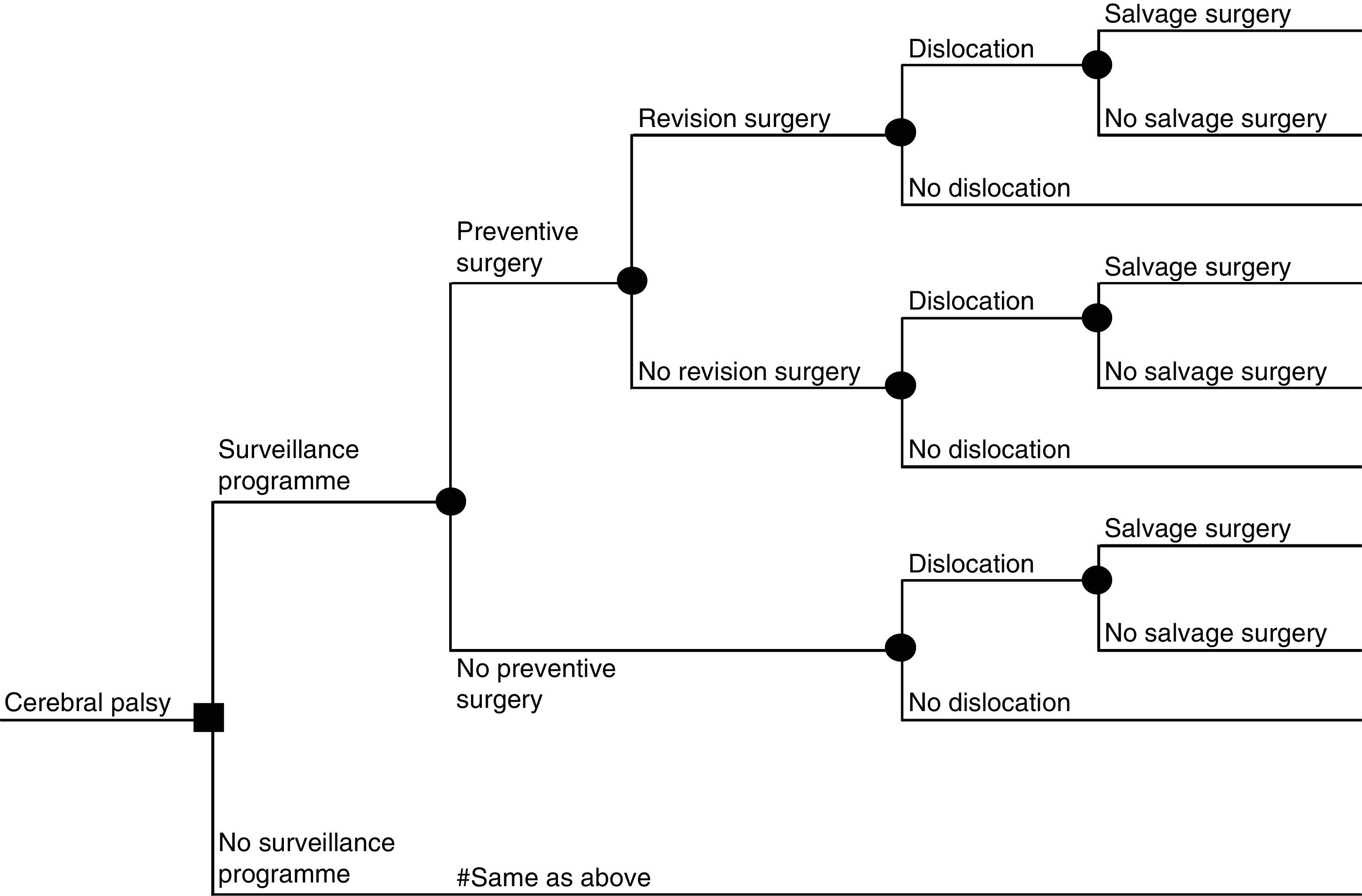
This is, to our knowledge, the first cost-effectiveness evaluation of a surveillance program to prevent hip dislocation in children with cerebral palsy. The analysis was thus based on a de novo decision analytical model. Similar to previous cost-effectiveness analyses of related interventions, such as screening of developmental dysplasia of the hip,18–20 the model took the form of a decision tree (Fig. 1). A decision tree is appropriate in this case because the data did not indicate a complex pattern of recurring-remitting health conditions. However, to account for the timing of preventive surgery, dislocation or salvage surgery, costs and QALYs associated with each pathway were computed on a yearly basis and summed up for the duration of the time horizon with appropriate discounting.
Based on this model, mean cost and mean QALYs and the incremental cost-utility ratio (ICUR) under both strategies were computed. The ICUR represents the additional cost required to achieve one additional QALY,16 which is then compared with the decision makers’ willingness to pay threshold in order to draw conclusions about the cost-effectiveness of the intervention. In Spain a cost-effectiveness threshold of 20,000-25,000€ per QALY has been recommended.21
Model input parameters- 1)
Effectiveness of the surveillance program
A systematic review was performed to identify studies that estimated the effectiveness of a screening program to prevent hip dislocation in children/adolescents (0 to 18 years old) with cerebral palsy. Methodological quality of the included studies was assessed with the Scottish Intercollegiate Guidelines Network (SIGN) criteria22 (see online supplementary Appendix I). The methods and results of the systematic review are described in detail in online supplementary Appendix II.
Three articles (reporting on two studies) were included in the final analysis. These two studies were retrospective analyses of the effectiveness of the same program: the Cerebral Palsy Follow-Up Program (CPUP), initiated in southern Sweden in 1994. Hägglund et al.,3,9 in 2005 and 2014, reported results at 10 and 20 years, respectively, comparing a cohort of children born between 1992-1997 (study group 1) and between 1998-2007 (study group 2) included in the CPUP, to a historical control group of children born in 1990-1991 not included in the program. Elkamil et al.,10 in 2011, compared a subsample of the CPUP to a sample of children recruited over the same period and with the same GMFCS (Gross Motor Function Classification System) levels in Norwegian regions who were not in a surveillance program. None of the studies reported data on pain or health-related quality of life.
The overlap of the intervention samples in the included studies precluded a meta-analysis of their results. The cost-utility model was populated based on data from Hägglund et al.,9 which provided the longest follow-up and compared two groups of children from the same region. The methodological quality of this study was evaluated as acceptable, the highest possible quality for retrospective studies according to the SIGN criteria (see online supplementary Appendix I).
In this study, children included in the control and intervention groups were not born in the same period. As a result, at the end of follow-up children in the control group (aged 22-23 years by then) have been at risk of developing dislocation (defined in the study as MP=100%) for far longer than children included in the intervention group 2 (aged 6-15 years at follow-up). Therefore, we compared information only from children included in intervention group 1 (aged 16 to 21 at follow-up) with those included in the control group, since they had a similar follow-up duration that was long enough to detect most cases of hip dislocation. Nine of 103 children developed dislocation of the hip in the control group (8 of them between 3 and 6 years of age, and one at age 16), while two out of 210 children from study group 1 included in the CPUP program suffered from a dislocated hip (relative risk=0.1090; 95% confidence interval [CI]: 0.2-0.49). Information on the probabilities of undergoing preventive primary, revision and salvage surgery were also computed by comparing children from study group 1 with the historical control cohort. These are presented in Table 1.
- 2)
Resource use and unit costs
Data inputs.
| Relative risk | Mean (95% CI) | Prob. distrib. | Ref. |
|---|---|---|---|
| Dislocation under surveillance program | 0.10899 (0.02-0.49) | Lognormal | 9 |
| Probabilities | Mean (SD) | Prob. distrib. | Ref. |
|---|---|---|---|
| Non-surveillance program | |||
| Primary preventive surgery | 0.1165 (0.0315) | Beta | 9 |
| Revision surgery after preventive surgery | 0.5833 (0.1367) | Beta | |
| Dislocation | 0.0874 (0.0277) | Beta | |
| Salvage surgery after dislocation | 0.4444 (0.1571) | Beta | |
| Surveillance program | |||
| Primary preventive surgery | 0.1512 (0.0223) | Beta | 9 |
| Revision surgery after preventive surgery | 0.4359 (0.0784) | Beta | |
| Salvage surgery after dislocation | 0.4444 (0.1571) | Beta |
| Proportion of type of preventive surgery | |||
|---|---|---|---|
| Tenotomy (vs. osteotomy) in primary preventive surgery | 0.6117 (0.0478) | Beta | 9 |
| Tenotomy (vs. osteotomy) in revision preventive surgery | 0.0652 (0.0360) | Beta | |
| Proportion of children in each group of the GMFCS | |||
|---|---|---|---|
| GMFCS I | 0.4312 (0.01791) | Dirichlet | 9 |
| GMFCS II | 0.1665 (0.01347) | Dirichlet | |
| GMFCS III | 0.1048 (0.01108) | Dirichlet | |
| GMFCS IV | 0.1442 (0.01270) | Dirichlet | |
| GMFCS V | 0.1533 (0.01303) | Dirichlet | |
| Unit costs | |||
|---|---|---|---|
| X-ray | 21.12 €(11.38 €) | Gamma | See online supplementary Appendix III |
| Physiotherapist visit | 25.84 € (18.17 €) | Gamma | |
| Occupational therapy visit | 20.17 € (7.29 €) | Gamma | |
| Orthopedic specialist doctor visit | 91.19 € (28.58 €) | Gamma | |
| Adductor–psoas tenotomy | 1,912.1 € (1,405.93 €) | Gamma | |
| Femoral osteotomy | 2,185.4 € (1,018.68 €) | Gamma | |
| Femoral resection | 3,253.4 € (948.07 €) | Gamma | |
| QALY weights | |||
|---|---|---|---|
| Cerebral palsy without hip dislocation(Mild cerebral palsy) | 0.8700 (0.2000) | Beta | 32 |
| Cerebral palsy with hip dislocation(Moderate cerebral palsy) | 0.7600 (0.2300) | Beta | |
| Disutility due to surgery (1-year) | 0.1000 (0.1000) | Beta | Assumption |
| Other parameters | Mean [min; max] | Prob. distrib. | Ref. |
|---|---|---|---|
| Age at primary preventive surgery | 5 [3;8] | Uniform | 9 |
| Age at revision surgery | 8 [4; 12] | Uniform | |
| Age at salvage surgery | 13 [7;20] | Uniform | |
| Age at dislocation | 5 [3;8] | Uniform |
CI: confidence interval; GMFCS: Gross Motor Function Classification System; Prob. distrib.: probability distribution; QALY: quality adjusted life years; SD: standard deviation.
Note: A more detailed description of the parameters summarized in Table 1 is provided in online supplementary Appendix V.
The intervention under analysis is the CPUP surveillance program,3,9 which included a standardized physiotherapist and occupational therapist visit twice a year until the age of six years, and once a year thereafter. Inclusion in the program was from identification of a possible cerebral palsy diagnosis, i.e., from birth on most patients, until they reached skeletal maturity. Radiological examinations in the CPUP program are based, since 2007, on the GMFCS, with children in level I not examined radiologically (if they have normal pain-free range of movement), children in level II examined at two and six years of age, and children in level III-V examined annually.9 GMFCS is currently the most widely applied scale for motor function classification in patients with cerebral palsy.23 We assume that each radiological examination involves a visit with an orthopeadic specialist doctor. In order to compute the mean cost of the program we considered the percentage of children in each GMFCS category as reported in Hägglund et al.,9 and shown in Table 1. This distribution by GMFCS was very similar to that reported in a previous study conducted in a Spanish region.24
In the CPUP, decisions regarding preventive surgery were made locally, and the most common types of preventive surgeries performed consisted of adductor–psoas tenotomy25 and varus femoral osteotomy.26 The proportions of surgery types are presented in Table 1, alongside with the mean age of children when undergoing preventive, revision and salvage surgery.
Information on the use of non-surgical treatments to prevent hip dislocation, such as appropriate lying, sitting and standing positions and the use of orthoses, is not provided in Hägglund et al.9 We assume there are not differences across groups in the provision of this usual care, and therefore these costs are not included in the analysis. Furthermore, no information was provided in this study on the follow-up required for children after surgery or who developed dislocation but could not undergo a surgical procedure. In our analysis we assumed, based on clinical expertise, an additional annual visit to the physiotherapist and an additional radiological examination involving an orthopaedic surgeon visit in these children. The impact of this assumption was tested in sensitivity analyses.
Unit costs data (Table 1) were taken from the mean values of the most up-to-date (2013 to 2018) Spanish regional tariffs (see online supplementary Appendix III for references).
- 3)
Life expectancy and health-related quality of life (HRQoL)
In order to calculate the QALYs associated to each strategy, we combined information on life-expectancy as well as on HRQoL, the latter expressed in terms of QALY weights.
We estimated mortality rates for patients with cerebral palsy until 18 years of age based on data from Hägglund et al.9 There is no evidence of differences in mortality for children under and not under a surveillance program, and neither there is evidence that hip dislocation has an impact on life expectancy in patients with cerebral palsy. Therefore, we applied the same mortality rates (represented as survival curves in online supplementary Appendix IV) for all children in our analysis.
Several studies have shown that hip displacement/dislocation is significantly associated with a lower HRQoL in children with cerebral palsy.27–30 However, these studies have used a measure of HRQoL not suitable for the computation of QALYs weights (e.g. the Child Health Index of Life with Disabilities). QALY measurement in paediatric populations is very challenging.31 One study by Carroll and Downs32 calculated QALY weights for a wide range of health problems in the paediatric population. They considered mild, moderate and severe symptoms for each health problem, including cerebral palsy. In our base case analysis, we applied the reported utilities for children with mild cerebral palsy to children with cerebral palsy not suffering from hip dislocation, and that estimated for children with moderate cerebral palsy to children with cerebral palsy with hip dislocation (Table 1). We explored the impact of this assumption in sensitivity analyses, and estimated the change in utility required for the program to be cost-effective. We assumed that the mean age of children developing dislocation was 5 years of age.3,9 Furthermore, to allow for the fact that undergoing a surgical procedure might have a short-term detrimental impact on HRQoL, we applied a disutility associated to any surgical procedure equivalent to 0.1 QALYs for one-year after surgery. The impact of this assumption is also analyzed in sensitivity analyses.
Sensitivity analysisData parameters were increased to double and reduced by half the base-case value in one-way deterministic sensitivity analyses. Wider ranges were applied to the assumptions included in the analyses: the number of follow-up visits after surgery was varied from no follow-up to monthly (base-case: annual visits); the disutility associated to any surgical procedure for a one-year after surgery was varied from 0.05 to 0.5 (base-case: 0.1); the time horizon of the study was varied from 10 to 100 years (base-case: 18 years) and discount rate varied from 1% to 5% (base-case: 3%). In addition, we undertook a threshold analysis that computed the value required on the change in QALY weights after dislocation for the intervention to be considered cost-effective.
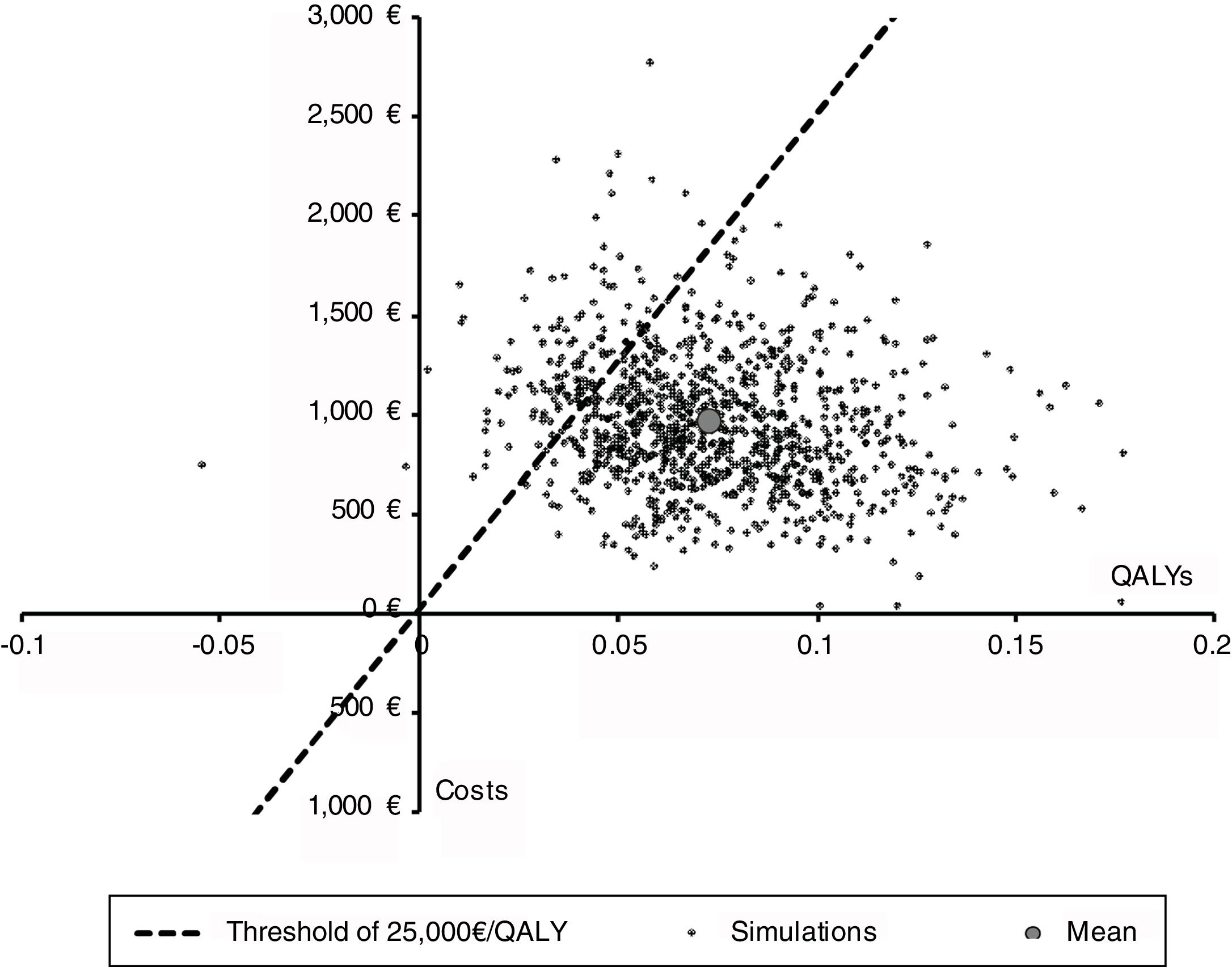
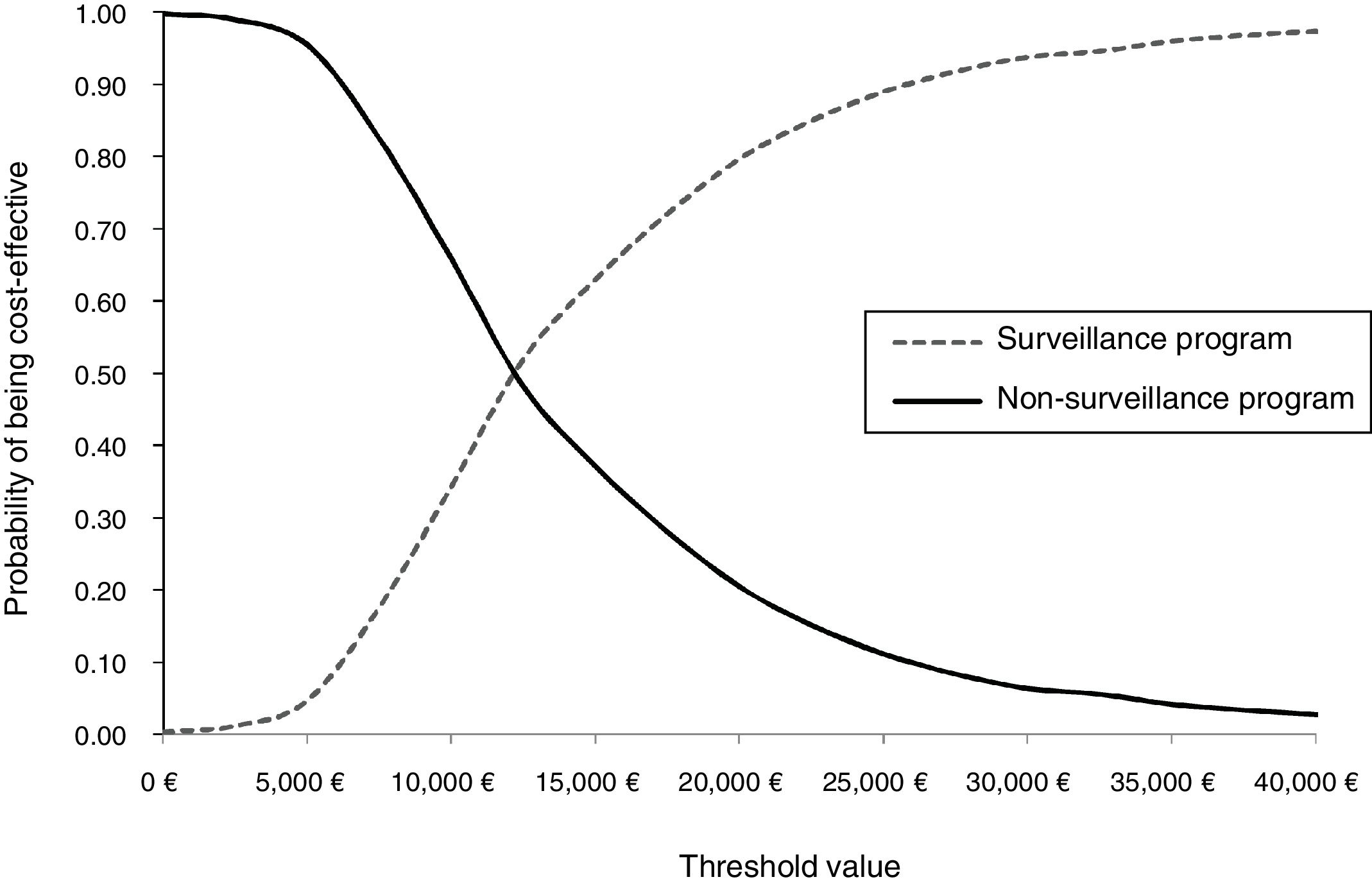
We also conducted a probabilistic sensitivity analysis to characterize the joint uncertainty in the model using 1,000 simulations in a Monte Carlo simulation. The results of the probabilistic sensitivity analysis are presented in terms of a cost-effectiveness plane and cost-effectiveness acceptability curves, which indicate the probability that an intervention is cost-effective for different values of the willingness to pay for a QALY. Probability distributions for each parameter are shown in Table 1.
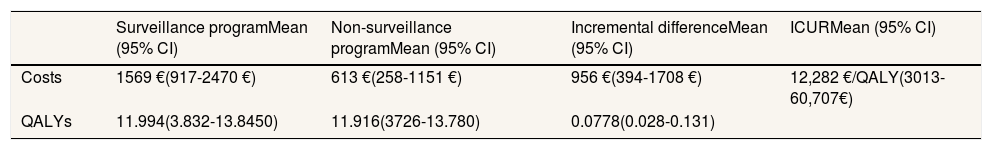
ResultsThe cost-utility results of the base case analysis are presented in Table 2. The mean cost per child included in the program is 1569€ (95% CI: 917-2470€) and the mean cost per child not included in the program is 613€ (95% CI: 258-1151€). The mean QALYs for children included and not included in the program are 11.99 (95% CI: 3.83-13.85) and 11.92 (95% CI: 3.73-13.78) QALYs, respectively, for an 18-year time horizon. The ICUR of the program is estimated as 12,282€/QALY (95% CI: 3014-60,708€), and therefore considerably lower than the threshold of up to 25,000/QALY recommended in Spain.21
Results for the base case analysis.
| Surveillance programMean (95% CI) | Non-surveillance programMean (95% CI) | Incremental differenceMean (95% CI) | ICURMean (95% CI) | |
|---|---|---|---|---|
| Costs | 1569 €(917-2470 €) | 613 €(258-1151 €) | 956 €(394-1708 €) | 12,282 €/QALY(3013-60,707€) |
| QALYs | 11.994(3.832-13.8450) | 11.916(3726-13.780) | 0.0778(0.028-0.131) |
ICUR: incremental cost-utility ratio; QALY: quality-adjusted life years.
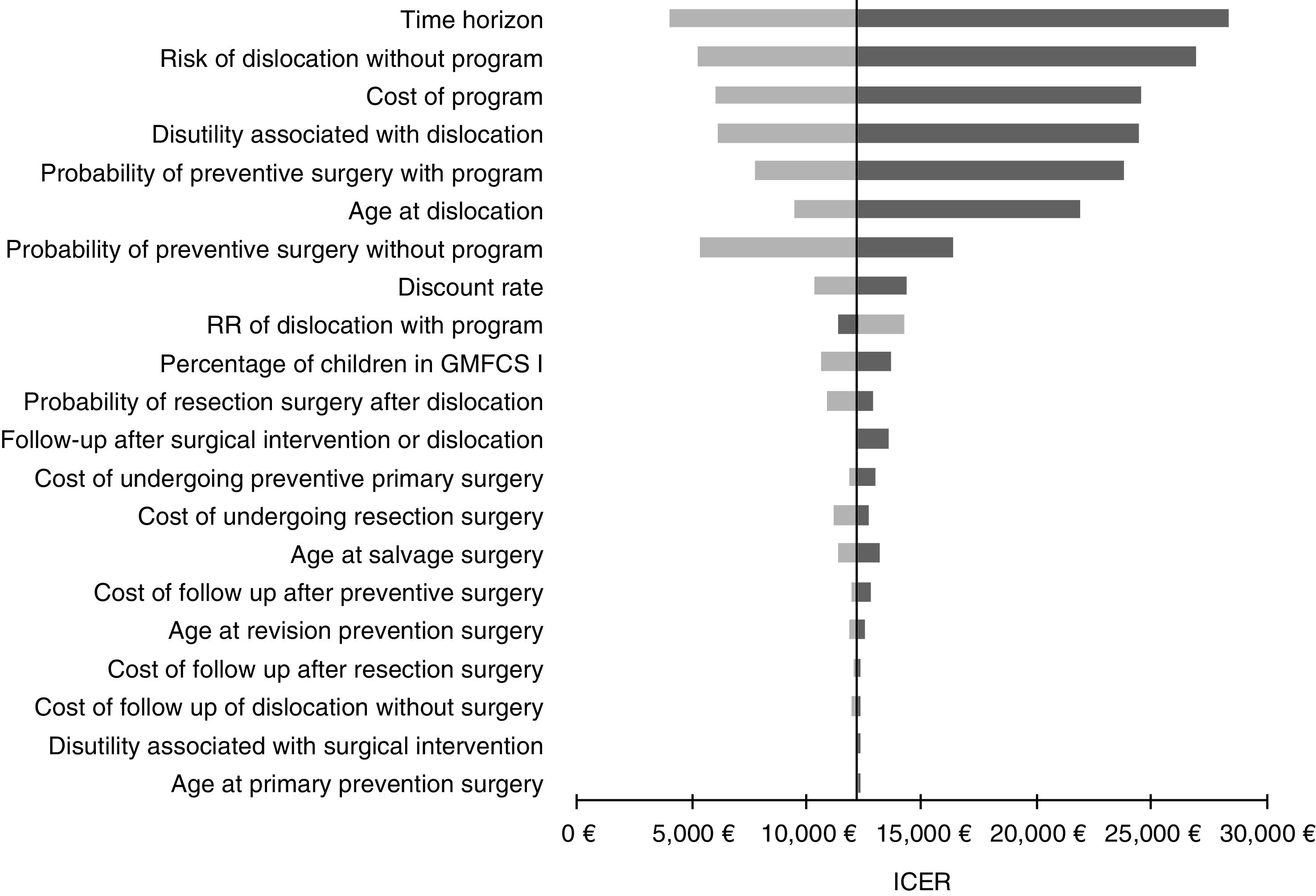
The results were robust to one-way sensitivity analyses (Fig. 2). The ICUR values estimated under the sensitivity analyses were in every instance under 25,000€/QALY, with the exception of when the time horizon is reduced to 10 years and when the underlying risk of dislocation without a program is assumed to be half the value of the base-case. Other variables found to have a large impact on the ICUR were the cost of the program, the probability of undergoing preventive surgery, and the disutility associated with dislocation. With regards to the latter, our analysis suggested that the screening program would be cost-effective when the difference in the utility weight between children with cerebral palsy with and without hip dislocation is 0.06 or greater (the base-case difference in the model is 0.11).
Figures 3 and 4 shows the cost-effectiveness plane and the cost-effectiveness acceptability curves. At a threshold value of €25,000/QALY, the probability that the surveillance strategy is the most cost-effective option approaches 90% in the base case analysis.
DiscussionThis study provides the first cost-utility evaluation of a surveillance program to prevent hip dislocation in children with cerebral palsy.
The analysis was based on the best available evidence, which is limited to a population-based retrospective observational study implemented in southern Sweden. This study showed acceptable internal validity (see online supplementary Appendix I). External validity might be compromised due to potential differences between countries in the organizational requirements to implement the program and the quality of services provided in usual care. Therefore, both the underlying dislocation rate under non-surveillance and the effectiveness of a surveillance program might be different in other contexts. Unfortunately, there are no data on the underlying dislocation rate in children with cerebral palsy in Spain, but similarly to the control group included in Hägglund et al.,9 current clinical guidelines for children with cerebral palsy in Spain do not include routine surveillance for hip dislocation, but only recommend considering annual radiological examinations in severe cases.33 Therefore, the rate of dislocation in Spain is unlikely to be lower than that reported in Hägglund et al.9 In fact, studies from other countries, such as in a Norwegian non-surveillance cohort with a 15 years of follow up,10 have reported a dislocation rate larger than that in the control group in Hägglund et al.9 (8.7% vs. 15.1%). Dislocation rates under surveillance programs in other countries have also being found to be larger to that observed in the intervention group of Hägglund et al.9 We identified three non-comparative studies of surveillance programs in Norway34 and Australia.14,35 The results of Connelly et al.14 and Terjesen et al.34 are not directly comparable to those of Hägglund et al.,9 since they defined dislocation as MP>90% instead of 100%, and indeed they reported much higher dislocation rates with shorter follow up (6.8% and 4%, respectively, vs. 1.0%). Wynter et al.35 published an abstract reporting ten years of follow up of the largest cohort studied to date (n=2278); they did not define dislocation, although in a previous 5-year report it was defined as 100% of MP.13 The observed dislocation rate in Wynter et al.35 (1.8%) was similar but slightly larger than that of Hägglund et al.9 (1.0%); although more than half of cases of dislocation were observed at the initial entry to the program. Another threat to external validity could be the technical evolution of therapeutic preventive and reconstructive procedures, since the CPUP program in Sweden started more than 20 years ago. However, treatment modalities have not fundamentally changed, as suggested by recent published systematic reviews about treatments options.8
The cost-utility analysis has a series of limitations. First, and related to the previous point, the analysis is based on data from the study implemented in Sweden and, therefore, some input parameters might not correspond to the epidemiological context and clinical practice in Spain. Nevertheless, the extensive sensitivity analyses conducted around these model parameters showed the results were generally robust to variations in these values. The validity of any model depends on a series of assumptions. In our model, these assumptions include the intensity of follow-up after surgery or when dislocation is not surgically manageable, as well as the disutility associated with surgical interventions. In every case results were found not to be sensitive to these assumptions. Finally, possibly the main methodological challenge in undertaking cost-utility analyses in paediatric populations pertains to the estimation of QALY weights. We explored the change required in the QALY weight of children with cerebral palsy with a dislocated hip for the intervention to be considered cost-effective, which was estimated in 0.06. This value is significantly lower than the baseline assumption. The results of previous papers that have shown a significant association of hip dislocation with a lower HRQoL in children with cerebral palsy,27–30 indicate that the program is cost-effective even when using conservative assumptions about HRQoL.
The results of this study suggest that a surveillance program to prevent hip dislocation in children with cerebral palsy is likely to be a cost-effective use of health care resources of the Spanish National Health System. However, there is a need for further research, in particular about epidemiological data on the incidence of dislocation in children with cerebral palsy, the impact of dislocation on quality of life, as well as on the comparative effectiveness of surveillance and other preventative treatments options.
Editor in chargeMiguel Ángel Negrín Hernández.
Transparency declarationThe corresponding author on behalf of the other authors guarantee the accuracy, transparency and honesty of the data and information contained in the study, that no relevant information has been omitted and that all discrepancies between authors have been adequately resolved and described.
Dislocation of the hip is a common but often preventable complication in children with cerebral palsy. Surveillance programs for early detection have been shown to be effective and practical, but no previous study has provided evidence on the cost-effectiveness of this intervention.
What does this study add to the literature?We provide the first cost-effectiveness evaluation of a surveillance program to prevent hip dislocation in children with cerebral palsy. Our study indicates that these programs are likely to be cost-effective. This information aims to support decision making in the Spanish National Health Service, but these results might be of relevance in other settings.
L. Vallejo-Torres, A. Rivero-Santana and L. Perestelo-Pérez conceived the study, and P. Serrano-Aguilar oversaw its conduct. A. Rivero-Santana, L. Perestelo-Pérez and P. Serrano-Aguilar conducted the systematic review of effectiveness. C. Martin-Saborido, C.L. Castellano-Fuentes and A. Escobar-Martínez contributed to the design of the model and to the data collection of the parameters required to populate the cost-effectiveness model, including epidemiological data, resource use, unit costs and utilities. L. Vallejo-Torres, A. Rivero-Santana and D. Epstein led the model design, analyzed the data and interpreted the results. L. Vallejo-Torres drafted the manuscript, and all authors edited and revised the manuscript, and approved the final manuscript.
FundingThis work was undertaken in the framework of activities run by the Network of Health Technology Assessment Agencies, funded by the Ministry of Health, Social Services and Equality in Spain.
Conflicts of interestNone.
AcknowledgementsThe authors would like to thank Carlos González Rodríguez for his support in the literature review.