To compare the incidence rates of gastric cancer among cancer survivors with those in the general population, and estimate the probability of a gastric second primary cancer being diagnosed 10 years after any other first primary cancer.
MethodA cohort of first primary cancers (other than gastric) diagnosed in Northern Portugal between 2000 and 2006 (n=64,648) was followed until 31/12/2012 for gastric second primary cancers. Incidence rates, standardized incidence ratios and the cumulative incidence of gastric second primary cancers were calculated.
ResultsOverall, 330 patients developed gastric second primary cancers (21.2% within two months). The incidence rate of gastric second primary cancers was higher within two months of the first primary cancer (standardized incidence ratios: 5.20 in males and 7.89 in females), particularly among survivors of cancers of the oesophagus, colon and rectum, than in the remaining period (standardized incidence ratios: 0.64 in males and 0.74 in females). The 10-year risk of a gastric second primary cancer was 0.6% (males: 0.7%; females: 0.4%).
ConclusionThe incidence rate of gastric second primary cancers among cancer survivors was higher than in the general population only soon after the first primary cancer, and lower thereafter. Despite the high mortality, the probability of a gastric second primary cancer within 10-years of the first primary cancer was 0.6%.
Comparar las tasas de incidencia de cáncer gástrico entre los sobrevivientes de cáncer con las de la población general y estimar la probabilidad de que se diagnostique un segundo cáncer primario gástrico 10 años después de cualquier otro primer cáncer primario.
MétodoSe siguió una cohorte de pacientes con un primer cáncer primario (excluyendo los gástricos) en el norte de Portugal entre 2000 y 2006 (n=64.648) hasta el 31/12/2012 para identificar un segundo cáncer primario gástrico. Se calcularon las tasas de incidencia, las razones de incidencia estandarizadas (RIE) y la incidencia acumulada de segundos cánceres primarios gástricos.
ResultadosEn total, 330 pacientes desarrollaron un segundo cáncer primario gástrico (21,2% en 2 meses). La tasa de incidencia de los segundos cánceres primarios gástricos fue mayor dentro de los 2 meses posteriores al primero (RIE: 5,20 en hombres y 7,89 en mujeres), en particular entre los sobrevivientes de cáncer de esófago, colon y recto, que en el período restante (RIE: 0,64 en hombres y 0,74 en mujeres). El riesgo a 10 años de un segundo cáncer primario gástrico fue del 0,6% (hombres: 0,7%; mujeres: 0,4%).
ConclusionesLa tasa de incidencia de segundos cánceres primarios gástricos entre los sobrevivientes de cáncer fue más alta que en la población general solo poco después del primer cáncer, y más baja a partir de entonces. A pesar de la alta mortalidad, la probabilidad de un segundo cáncer primario gástrico a 10 años del primero fue del 0,6%.
The number of cancer survivors is growing, reflecting increases in the overall number of cancer cases and improvements in prognosis, as a result of early diagnosis as well as advances in treatment.1,2 These survivors are at increased risk of several long-term and late adverse health events including the recurrence of the first primary cancer (FPC), cardiovascular diseases and second primary cancers (SPCs).3 After an FPC diagnosis, a greater risk of an SPC could be expected, due to increased genetic susceptibility, persistence of exposures or effects of environmental risk factors, or a late result of the treatment of the FPC.4
SPCs account for approximately 10% of all cancer diagnoses in Western countries.3,5 In Northern Portugal, a recent population-based study showed that cancer survivors had a 31% increased incidence rate of SPC relative to the general population.6 In particular, gastrointestinal cancers accounted for a large part of SPCs, with gastric cancer representing nearly one out of each 10 SPCs diagnosed.7 Additionally, in Portugal, gastric cancer ranks fifth in incidence and mortality,8 with much higher estimates being observed in the North.9,10
In the present study, we followed a population-based cohort of cancer survivors from the North of Portugal for the occurrence of gastric SPCs. Our aim was to calculate the incidence rates of gastric SPCs and the corresponding standardized incidence ratios (SIRs), and to estimate the probability of being diagnosed with a gastric SPC up to 10-years after any other FPC.
MethodsStudy settingWe conducted a cohort study based on the North Region Cancer Registry (RORENO). This population-based cancer registry was established in 1988, and currently covers approximately one-third of the Portuguese population, i.e. over three million people. RORENO follows the cancer registration principles and methods of the International Agency for Research on Cancer (IARC), and data are regularly checked with pre-defined algorithms for validity and consistency.11 The registry fulfilled IARC indices of data quality from 1998 to 2002, which indicates a high degree of completeness of ascertainment.12,13
RORENO collects routine demographic and clinical data in accordance with the Portuguese Privacy Policy and with approval from the Portuguese Data Protection Authority. Individual patient consent is not required for registry data. The registry was responsible for creating a database with the relevant personal and clinical information, and as part of the routine activity by the registry, patient follow-up was accomplished. For data analyses, the database was anonymized by removing personal information. In this way, patients were not identified or identifiable with the information available in the database, which was used for research purposes only. Further, the study was approved by the Ethics Committee of the Portuguese Institute of Oncology of Porto (Ref. CES IPO: 173/2015).
Tumour classification and definition of second primary cancersTumour topography and morphology were classified according to the International Classification of Diseases for Oncology, 3rd edition,14 and further recoded as per the International Statistical Classification of Diseases and Related Health Problems, 10th revision.15
SPCs were defined as a new cancer in a person with a history of malignancy.16 Multiple primary cancers were defined according to the guidelines proposed by the International Association of Cancer Registries and IARC.17 Primary cancers are those that originally develop in an organ or tissue, not being an extension, recurrence or metastasis. Different morphologies (even with the same topography) or dissimilar topographies should be regarded as multiple primary cancers, regardless of the time between diagnoses, unless they correspond to systemic cancers, which are considered the same cancer.
Study designThe study cohort included all adult Northern Portuguese residents diagnosed with an FPC (other than gastric) between 1 January 2000 and 31 December 2006 (n=66,060). The vital status of patients was obtained through record linkage with the National Health System database using the patients’ National Health System card number; when this number was not available, the name and/or date of birth were used. Among the latter, the available information did not always yield a unique identifier for linkage with the National Health System for assessment of vital status, resulting in 1412 patients being excluded; these were significantly different from those included regarding sex (men: 67.7% vs. 54.7%, respectively; p <0.001), age at FPC diagnosis (median: 69.7 vs. 65.2, respectively; p <0.001), FPC site (e.g. prostate: 30.2% vs. 14.4%; colon: 15.0% vs. 11.1%; rectum: 9.3% vs. 6.5%; and female breast: 7.2% vs. 14.1%, respectively; p <0.001) and year of FPC diagnosis (e.g. 2000: 20.8 vs. 11.8%, respectively; p <0.001).
The remaining patients (n=64,648) were followed to 31 December 2012, until a gastric SPC or death, whichever occurred first. The diagnosis of gastric SPCs was ascertained through record linkage with the list of cases registered by RORENO. Patients known to have died but with an unknown date of death were imputed a follow-up time equal to the median follow-up of the corresponding group, defined by sex, age (<55, 55-64, 65-74 and ≥75 years), FPC site and year of diagnosis (n=194); when there were less than 25 cases per year, the imputed follow-up was the observed for the corresponding FPC site (n=4).
We classified gastric SPCs as synchronous when diagnosed simultaneously or within two months of the FPC, or metachronous otherwise.3,7,18 For patients who had a non-gastric SPC before a gastric SPC or a non-gastric SPC following a gastric SPC, only the FPC and gastric SPC were considered.
Statistical analysisThe characteristics of patients were presented as counts and proportions for categorical variables, and median (percentile 25-percentile 75 [P25-P75]) for continuous variables. To compare continuous and categorical variables across groups, the Mann-Whitney test and Chi-square test were used, respectively. Median follow-up time was estimated using the reverse Kaplan-Meier.19 Statistical significance was considered when p <0.05. All p-values were two-sided. Analyses were carried out for all gastric SPCs, and separately for synchronous and metachronous SPCs.
Person-years at risk (PYAR) were calculated as the time from FPC diagnosis to the gastric SPC diagnosis, death or end of follow-up (31 December 2012), whichever occurred first. The incidence rate of gastric SPCs for males and females was computed for different follow-up periods (“0 to <1 month” from FPC diagnosis to less than one month; “≥1 to <2 months” from one to less than two months; “≥2 to <4 months” from two to less than four months; “≥4 to <6 months” from four to less than six months; “≥6 months to 1 year” from six months to less than one year; and then for each year), by dividing the number of incident gastric SPCs by the PYAR within each time interval.
The incidence of gastric SPCs was compared with age-, sex-, and calendar year-specific rates in the general population from Northern Portugal, by calculating SIRs. These were calculated by dividing the observed number of gastric SPCs by the expected number of cases, in the same time period, if the gastric cancer incidence rates in the general population had been observed among cancer survivors. The latter were estimated by multiplying the gastric cancer incidence in the general population by the PYAR in the corresponding stratum defined according to sex, 5-year age group (from 15-19 to 70-74, and ≥75 years) and calendar year (2000-2012). The incidence of gastric cancer among the general population was acquired from RORENO.20 The 95% confidence intervals (95%CI) of the SIRs were estimated assuming that the observed number of cancers followed a Poisson probability distribution. SIRs and the corresponding 95%CIs were also estimated for two different periods: “0-2 months” from FPC diagnosis to two months of follow-up, and “>2 months” from two months to the end of follow-up.
Cumulative incidence and corresponding 95%CI, stratified by sex, age and the most frequent FPC site, were calculated, for all gastric SPCs and specifically for metachronous gastric SPCs, considering death as a competing event according to the method of Kalbfleisch and Prentice.21
Statistical analysis was conducted using STATA® 11 (StataCorp. 2009. College Station, TX).
ResultsAmong 64,648 patients with an FPC other than gastric cancer diagnosed between 2000 and 2006, 330 (0.5%) developed a gastric SPC during follow-up, from which 70 (21.2%) were diagnosed within the first two months of the FPC.
Table 1 shows patients’ characteristics. Gastric SPCs occurred more often in men (p <0.001). Those who developed a metachronous gastric SPC were significantly older (median [P25-P75]: 67.1 [56.8-73.3] vs. 65.2 [53.5-73.8]; p=0.011). The most common FPC sites were digestive organs (n=129, 39.1%), from which over half were classified as metachronous tumours (n=69, 53.5%). The distribution of tumour location within the stomach was similar between patients with a synchronous or metachronous SPC (p=0.178).
Features of cancer patients with synchronous and metachronous second gastric primary cancers, and without a second gastric primary cancer.
| Total | Patients withouta gastric SPC | Patients with a gastric SPC | ||
|---|---|---|---|---|
| N=64,648 (100.0%) | N=64,318 (99.5%) | N=330 (0.5%) | ||
| SynchronousN=70 | MetachronousN=260 | |||
| Sex, n (%) | ||||
| Males | 35373 (54.7) | 35153 (54.7) | 45 (64.3) | 175 (67.3) |
| Females | 29275 (45.3) | 29165 (45.3) | 25 (35.7) | 85 (32.7) |
| Age at FPC diagnosis, median (P25-P75) | 65.2 (53.5-73.8) | 65.2 (53.5-73.8) | 68.4 (58.2-75.9) | 67.1 (56.8-73.3) |
| <45 years | 7933 (12.3) | 7909 (12.3) | 6 (8.6) | 18 (6.9) |
| 45-54 years | 9987 (15.4) | 9947 (15.5) | 8 (11.4) | 32 (12.3) |
| 55-64 years | 14064 (21.8) | 13991 (21.8) | 13 (18.6) | 60 (23.1) |
| 65-74 years | 18660 (28.9) | 18535 (28.8) | 24 (34.3) | 101 (38.8) |
| ≥75 years | 13978 (21.6) | 13910 (21.6) | 19 (27.1) | 49 (18.8) |
| FPC site, n (%)a | ||||
| Oesophagus | 1191 (1.8) | 1166 (1.8) | 18 (25.7) | 7 (2.7) |
| Colon | 7107 (11.0) | 7027 (10.9) | 22 (31.4) | 37 (14.2) |
| Rectum | 4133 (6.4) | 4104 (6.4) | 9 (12.9) | 20 (7.7) |
| Trachea, bronchus and lung | 5975 (9.2) | 5954 (9.3) | 7 (10.0) | 14 (5.4) |
| Female breast | 9153 (14.2) | 9124 (14.2) | 0 (0.0) | 29 (11.1) |
| Prostate | 9075 (14.0) | 9013 (14.0) | 0 (0.0) | 62 (23.8) |
| Gastric SPC tumour location, n (%)b | ||||
| Cardia | 9 (12.9) | 22 (8.5) | ||
| Non-cardia | 17 (24.3) | 91 (35.0) | ||
| Gastric NOS | 44 (62.9) | 147 (56.5) | ||
| Follow-up, years, median (P25-P75)c | 9.0 (7.5-10.8) | 9.1 (7.5-10.8) | 0.05 (0.03-0.09) | 5.5 (2.9-7.4) |
| Dead, n (%)d | 35227 (54.5) | 34990 (54.4) | 58 (82.9) | 179 (68.8) |
Note: In each variable, the total may not add up due to missing data.
FPC: first primary cancer; NOS: not otherwise specified; P25: percentile 25; P75 percentile 75; SPC: second primary cancer.
Oesophagus (C15), colon (C18), rectum (C19-C20), trachea, bronchus and lung (C33-C34), female breast (C50), prostate (C61) defined according to the International Statistical Classification of Diseases and Related Health Problems 10th Revision.15
Defined as cardia (C16.0), non-cardia (fundus, C16.1; body, C16.2; pyloric, C16.3, pylorus, C16.4; lesser and greater curvature, C16.5-6) and other parts (C16.8-9).15
Follow-up to 31 December 2012 until SPC, death or end of follow-up. Median follow-up time was estimated using the reverse Kaplan-Meier.19
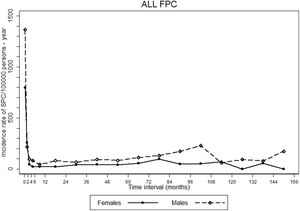
The incidence rate of gastric SPCs was more than nine-fold higher in the first two months of follow-up than in the remaining period, after which incidence rates remained relatively stable (Fig. 1).
Trends in incidence rates of gastric second primary cancers (SPC) since the diagnosis of the corresponding first primary cancers (FPC). Incidence rates were estimated for and represented in the respective midpoint of the following intervals: 0 to <1 month, ≥1 to <2 months, ≥2 to <4 months, ≥4 to <6 months, ≥6 months to 1 year, and then for each year of follow-up.
The most frequent FPCs were prostate (n=62, 28.2%), colon (n=32, 14.5%) and oesophagus (n=19, 8.6%) among men, and breast (n=29, 26.4%), colon (n=27, 24.5%) and rectum (n=13, 11.8%) in women. All gastric SPCs in prostate and breast cancer survivors among men and women, respectively, were diagnosed more than two months following the FPC (Fig. 2).
Standardized incidence ratios (SIR) and 95% confidence intervals (95%CI) for the diagnosis of a second gastric primary cancer, according to the first primary cancer and follow-up time since its diagnosis, among males (A) and females (B). First primary cancer: oesophagus (C15), colon (C18), rectum (C19-C20), trachea, bronchus and lung (C33-C34), female breast (C50), prostate (C61) defined according to the International Statistical Classification of Diseases and Related Health Problems 10th Revision.15
The incidence rate of gastric SPCs observed among male cancer survivors was 22% lower than in the general population (SIR [95%CI]: 0.78 [0.68-0.89]). This lower SIR was mainly identified for metachronous gastric SPCs (SIR [95%CI]: 0.64 [0.55-0.74]), while a SIR higher than one was found when considering the first two months of follow-up (SIR [95%CI]: 5.20 [3.79-6.96]) (Fig. 2). Considering synchronous gastric SPCs, the highest SIRs were observed for the oesophagus, colon, rectum, and trachea, bronchus and lung; a SIR significantly higher than one remained for the oesophagus when considering metachronous SPCs, while a SIR lower than one was observed for the colon and rectum. With regards to prostate cancer survivors, the incidence rate of developing a gastric SPC was lower than the expected in the general population.
Among female cancer survivors, there was also a SIR significantly higher than one of developing a gastric SPC within two months of the FPC (SIR [95%CI]: 7.89 [5.11-11.65]), while a SIR lower than one was found when considering metachronous gastric SPCs (SIR [95%CI]: 0.74 [0.59-0.91]) (Fig. 2). Gastric SPCs had the highest SIRs for oesophagus, colon, and trachea, bronchus and lung FPCs when considering the total follow-up time and within the first two months of the FPC. No statistically significant results were observed for these FPCs when considering metachronous SPCs. Concerning breast cancer survivors, the incidence rate of developing a gastric SPC was lower than that of the general population.
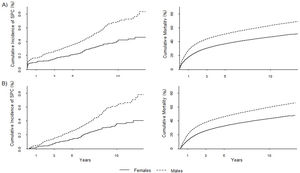
Cumulative incidence of gastric second primary cancers and deathFigure 3 depicts the overall 10-year cumulative incidence of gastric SPC, which was 0.6% (males: 0.7%; females: 0.4%; p <0.001); the corresponding 10-year cumulative mortality was 55.9% (males: 63.4%; females: 46.9%; p <0.001). When considering metachronous SPCs alone, the 10-year risk was 0.5% (males: 0.6%; females: 0.4%; p <0.001), with a respective risk of death of 52.0% (males: 59.6%; females: 43.2%; p <0.001). Among both genders, oesophageal FPCs showed the highest cumulative incidence of gastric SPCs; however, when only metachronous SPCs were considered, in men, prostate FPCs had the highest estimates, while in women, the oesophagus persisted (see Tables I and II of the online Appendix of this article).
DiscussionThe incidence rate of a gastric SPC was much higher in the first months after the FPC diagnosis than in the remaining period. Compared to the general population, cancer survivors had significantly higher incidence rates of gastric SPC within the first two months of the FPC; this increase remained when considering FPC survivors of digestive organs. On the other hand, breast and prostate FPC survivors had significantly lower incidence rates of a gastric SPC than the general population. The overall 10-year risk of gastric SPCs and death were 0.6% and 55.9%, respectively.
Although many studies have analysed SPCs, few have specifically examined the incidence of gastric SPCs. The proportion of gastric SPCs diagnosed in cancer survivors in Northern Portugal was higher than in Sweden, where over 2,500 (0.3%) gastric SPCs were reported in 824,465 individuals with an FPC diagnosed from 1958 to 1998 and followed to December 31, 2002.22 On the other hand, our estimate is lower than that from Japan, where 2,209 (1.3%) gastric SPCs were observed in more than 170,000 cancer patients diagnosed from 1985 to 2007 and followed to the end of 2008.23 Although both of these studies have a longer follow-up period than our study, differences in the proportion of gastric SPCs diagnosed naturally reflect the overall incidence of gastric cancer in each country.8
Previous studies have compared the incidence of gastric SPCs with that of the general population: in Sweden, SIRs significantly higher than one after cervical, ovarian and testicular cancers, were observed.22 However, these estimates were adjusted for sex, age, period, residence, as well as socioeconomic level, which were not all taken into account in our study. A study from Australia found that only female breast cancer survivors were more likely to be diagnosed with gastric cancer than the general population.18 In Japan, patients diagnosed with any FPC had SIRs of a gastric SPC higher than one.23 Similar to our results, they also found that oesophageal and colorectal cancer survivors had a SIR higher than one of developing a gastric SPC, while males with a prostate FPC had a SIR lower than one.23 A population-based study on SPCs in Germany and Sweden between 1997 and 2006 found that gastric cancer was the fifth most common SPC among German colorectal, prostate and bladder cancer survivors.24
Generally, there is an immediate excess of a gastric SPC following any cancer due to enhanced medical surveillance or similarity of diagnostic procedures in patients.3,25 This is in line with our finding of the highest SIRs among both genders in patients with digestive FPCs and also in accordance with a previous study which found that oesophageal FPCs had a SIR of gastric SPC higher than one within the first year after diagnosis.22 Moreover, this may suggest shared risk factors (including diet, alcohol intake and smoking) and the common carcinogenic process that affect the gastrointestinal tract.26,27
We observed a significantly lower incidence of a gastric SPC following a breast or prostate cancer diagnosis than in the general population. This may be due to the fact that they have no major risk factors in common with gastric cancer.28 Furthermore, individuals with higher socioeconomic level have lower gastric cancer incidence than those with lower socioeconomic level,29 whereas the incidence of breast and prostate cancer increases with higher socioeconomic level.30
Although the sample was obtained from RORENO, which is representative of Northern Portuguese cancer survivors, our results on the incidence of gastric SPCs may not be representative of all of Portugal as the North has higher incidence rates of gastric cancer compared to the rest of the country,9 thus extrapolating our results to the rest of the country should be done cautiously. However, the use of a population-based cancer registry allowed us to follow over 60,000 cancer survivors for the occurrence of a gastric SPC to obtain accurate cumulative incidence and SIR estimates. Less than 3% of patients were lost to follow-up as they could not be linked to the National Health System database due to a missing National Health System card number, and when the use of the name and/or date of birth of cancer survivors did not yield a unique identifier precluding linkage with the National Health System database to follow all patients. Despite the statistically significant differences between included and excluded patients, we do not expect this to significantly impact our results since these differences were relatively small and less than 3% were excluded. Although we imputed follow-up for some patients who had an unknown date of death, we took into account sex, age, FPC site and year of diagnosis, which influence survival.31 As such, we do not believe that this would meaningfully misclassify PYAR. Additionally, we were limited by the data available in RORENO and though our SIR estimates were age-adjusted, and stratified results by gender and the most common FPC sites for gastric SPCs are provided, we were unable to quantify the contribution of socioeconomic level, family history and lifestyle (diet, alcohol and smoking), which have been shown by some studies to be associated with higher than expected SPCs among certain FPCs.22,32,33 Finally, we could not assess the role of stage at FPC diagnosis as complete data on the stage of tumours are not available from RORENO. We would expect patients with less advanced stages at diagnosis to have higher incidence rates of gastric SPC compared to the general population, due to a lower competitive risk from death.
Overall, our results demonstrate that cancer survivors in Northern Portugal have a lower incidence rate of gastric SPC than the experienced by the general population. However, significantly higher incidence rates of a gastric SPC within the first two months following an FPC diagnosis were observed and higher incidence rates remained for other digestive FPCs. Despite the high mortality, the probability of a gastric SPC within 10 years of the FPC was 0.6%. As such, these findings suggest the need for further research to more accurately characterize the burden of gastric SPCs among the growing population of cancer survivors in different contexts. Additionally, this highlights the need of encouraging healthy lifestyles to potentially prevent the occurrence of gastric SPCs in the long-term, as well as assessing the impact of close surveillance of cancer survivors for early detection and treatment of gastric SPCs in order to reduce their morbidity and mortality burden.
Second primary cancers are increasing among cancer survivors. In Northern Portugal, gastric cancer incidence is high representing 10% of all second primary cancers diagnosed. Accounting for the competing risk of death is necessary for accurately quantifying the occurrence of second primary cancers.
What does this study add to the literature?Cancer survivors in Northern Portugal have a lower incidence rate of gastric second primary cancers than the experienced by the general population with a 10-year cumulative incidence of 0.6%. This is an accurate estimation of risk of second primary cancers, which is important to plan health services and manage the expectations of cancer survivors.
María-Victoria Zunzunegui.
Authorship contributionsS. Morais and N. Lunet defined the manuscript hypotheses and designed the investigation. S. Morais collected data, performed the statistical analysis and interpretation of results, and drafted the manuscript. L. Antunes and M.J. Bento supplied the data and revised the manuscript for relevant intellectual content. NL supervised the analysis and interpretation of data, and reviewed the manuscript. All authors read and approved the final version of the manuscript.
FundingThis study was funded by FEDER through the Operational Programme Competitiveness and Internationalization and national funding from the Foundation for Science and Technology – FCT (Portuguese Ministry of Science, Technology and Higher Education), under the Unidade de Investigação em Epidemiologia - Instituto de Saúde Pública da Universidade do Porto (EPIUnit) (POCI-01-0145-FEDER-006862; Ref. UID/DTP/04750/2013). An individual PhD grant attributed to S. Morais (SFRH/BD/102585/2014) was funded by FCT and the “Programa Operacional Capital Humano” (POCH/FSE).
Conflicts of interestNone.