The ITC EUREST-PLUS Spain Survey is a longitudinal study of a representative sample of Spanish adult (≥18 years old) smokers. This protocol describes the methods of the 2021 follow-up survey.
MethodThe ITC EUREST-PLUS Survey, a prospective cohort study of a representative sample of smokers in six European countries, was conducted in 2016 (baseline) and 2018 (waves 1 and 2). The 2021 ITC EUREST-PLUS Spain Survey is a continuation of the Spanish cohort with a new interview in 2021 (wave 3). Lost participants were replaced with new smokers recruited using the same multi-stage sampling design. This latest follow-up aims to examine current patterns and transitions of tobacco use and to evaluate the impact of new tobacco-related policies.
CommentsThe ITC EUREST-PLUS Spain Survey will provide recent information about the impact of tobacco control policies on smoking behaviour.
La encuesta ITC EUREST-PLUS España es un estudio longitudinal de una muestra representativa de fumadores adultos (≥18 años) españoles. Este protocolo describe los métodos de la encuesta de seguimiento de 2021.
MétodoLa encuesta ITC EUREST-PLUS, un estudio de cohortes prospectivo de una muestra representativa de fumadores en seis países europeos, se realizó en 2016 y 2018 (olas 1 y 2). La encuesta ITC EUREST-PLUS España de 2021 es una continuación de la cohorte española con una nueva entrevista en 2021 (ola 3). Se sustituyeron las pérdidas con nuevos fumadores reclutados usando el mismo diseño muestral multietápico. Este último seguimiento pretende examinar los patrones actuales y las transiciones del uso de tabaco, y evaluar el impacto de nuevas políticas relacionadas con el tabaco.
ComentariosLa encuesta ITC EUREST-PLUS España de 2021 proporcionará información reciente sobre el impacto de las políticas de control del tabaco en el consumo de tabaco.
Tobacco smoking is the leading cause of preventable morbidity in Europe.1 In 2005, the European Union (EU) ratified the World Health Organization Framework Convention on Tobacco Control (WHO FCTC).2–4 Similarly, in 2014 the European Parliament approved the Tobacco Products Directive (TPD), requiring the EU Member States to incorporate it into national law by 2016. Both strategies aim to tackle some of the causes of the tobacco epidemic. While the WHO FCTC guides Parties in incorporating recommended policies and measures into national legislation, including tobacco product taxation, comprehensive smoke-free laws, tobacco advertising, promotion and sponsorship, and support for tobacco cessation services, the TPD regulates the products themselves, including their ingredients, additives, packaging, labelling, and reporting of novel tobacco products.5–7
The International Tobacco Control Six European Countries (ITC 6E) Survey, part of the EUREST-PLUS Project,8 aimed to monitor and evaluate the psychosocial and behavioural impact of the TPD by establishing a cohort of adult smokers in Germany, Greece, Hungary, Poland, Romania, and Spain.9 This cohort is part of the ITC Project founded at the University of Waterloo in 2002.10 The cohort was established in 2016 (ITC 6E1) and recontacted in 2018 (ITC 6E2). A third wave was conducted only in Spain in 2021 (ITC ES2.5). The six-country objective of this article is to describe the protocol of this third wave in Spain.
MethodStudy designAll respondents from the 2018 wave were invited to participate in the 2021 Survey. Respondents lost to follow-up were replaced with new respondents, using the same sampling design as in the previous surveys.9
At baseline, multistage sampling was used with geographical stratification, including all Spanish regions, except the Canary Islands, Ceuta, and Melilla. The design was multistage within strata, which were crossed with the degree of urbanisation (urban, intermediate, rural). The strata were conceptually divided into clusters, each the size of an enumeration area. A sample of 100 clusters was allocated to the strata proportionally to population size, requiring at least two clusters per stratum; households within clusters were sampled using the random route approach. A starting point was randomly selected using GPS coordinates, and a random walk path was pre-drawn to approach every fifth address for household screening. In case of multiple households, a single household was randomly selected. Where possible, one male and one female smoker per household were randomly selected using the last birthday method. Household screening continued until approximately 10 smokers per cluster were interviewed.
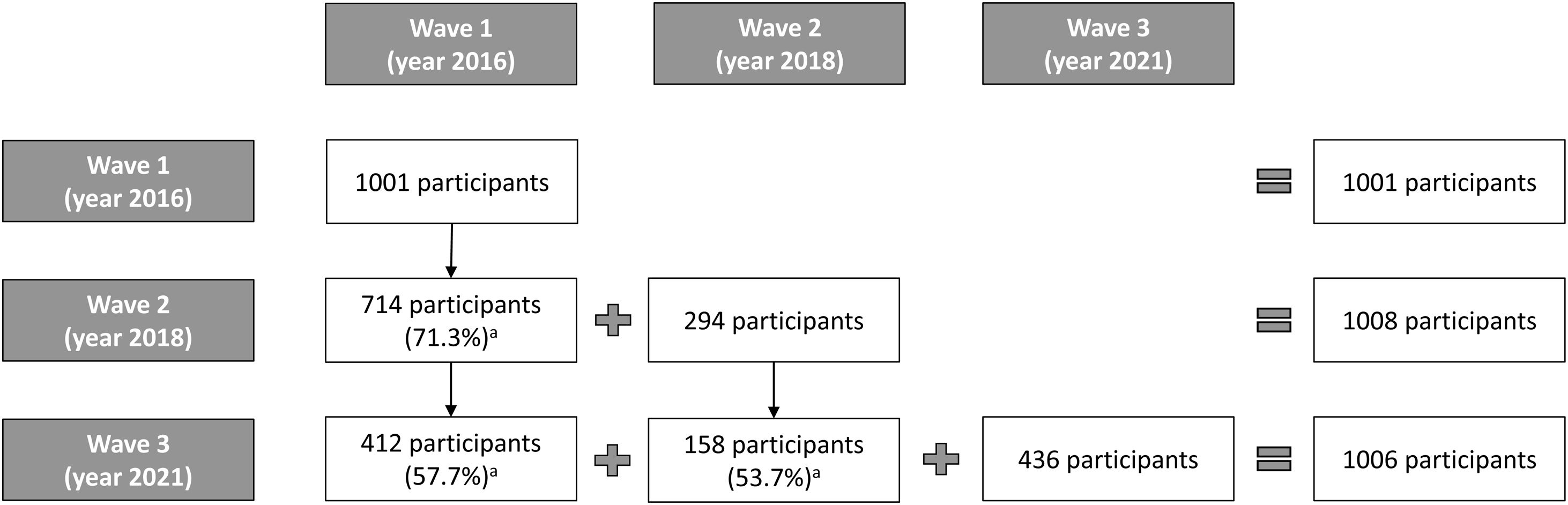
Of the 887 participants in 2018 who agreed to be recontacted in the future, 570 (64.3%) completed the Wave 3 survey in 2021. Respondents lost to attrition were replaced with new respondents, selected from new households screened in the same clusters, using the methodology described above. Respondents were 18 years and older, smoked at least monthly, and had smoked at least 100 cigarettes in their lifetime.
Several measures were taken to minimise losses to follow-up. Respondents were visited at home on different days and at different times. Wherever possible, the same interviewers from previous waves contacted the same participants. A token of appreciation was given on survey completion. Respondents were also asked if they would be willing to be contacted again for further follow-up.
Ethical considerationsThe EUREST-PLUS Spain Project received ethics clearances from the Research Ethics Boards of the Bellvitge University Hospital, Spain (PR100/16) and the University of Waterloo, Canada (REB#41105). Respondents to the 2018 survey received an invitation letter explaining the study, data confidentiality and security, and the potential risks and benefits of participation. New respondents received this information in person. All respondents gave consent to participate.
Adaptation to the COVID-19 pandemicThe COVID-19 pandemic required some adjustments to the survey protocol. Fieldwork was delayed, and special protocols were developed for face-to-face interviews to reduce the risk of infection. A risk assessment introduction was developed in which interviewers and all potential respondents were asked to confirm that they were not currently infected and had not been in contact with an infected person in the previous 14 days. Social distancing, masking, and hygiene measures were implemented. In addition to the usual computer-assisted personal interviewing (CAPI), a modified computer-assisted telephone interviewing (mCATI) was developed to allow all respondents to participate by telephone if they preferred it.
Survey weightingAfter data processing and cleaning, survey weights were constructed. To estimate the probability of being a smoker in each stratum, smoking prevalence was modelled using the 2014, 2017, and 2020 Eurobarometer surveys for each wave. Projections from Eurostat population censuses for 1 January 2015, 2017, and 2021 were also used to estimate the number of people in each stratum.
Cross-sectional and longitudinal weights were constructed for Waves 1-3 and 2-3. These weights were calibrated to be nationally representative at baseline by stratum/sex/age group, where appropriate.11
Instrument and variablesThe Wave 3 questionnaire is a revision of the Wave 2 version.12 The main domains covered were:
- •
Smoking behaviour: smoking characteristics, quit attempts, cessation aids received, beliefs about quitting smoking, and exposure to second-hand smoke (SHS).
- •
Tobacco products used and product perceptions: preferences for specific tobacco/nicotine products, brands used, purchasing sources, reasons for their preferences, knowledge and perceptions of the health effects of smoking, and psychological beliefs about smoking.
- •
Impact of tobacco control policies: prices and taxes, smoke-free environments, health warnings, plain packaging, anti-smoking campaigns, tobacco advertising and promotion, smoking cessation, and other policy support issues.
- •
COVID-19 pandemic: perceived impact of the pandemic on smoking behaviour and SHS exposure during lockdown, perceptions of the disease, and social behaviours related to social distancing and smoking restrictions in open public places.
- •
Health status and demographics: comorbidity, psychological stress and socio-demographics.
The fieldwork was conducted between 9 June and 5 August 2021. All respondents in 2018 were first sent an information letter by post, followed by a telephone call; those who were unavailable were visited at home. New respondents were contacted at home. Interviews were conducted in-person (CAPI) or by telephone (mCATI) if they had been recently tested positive, had symptoms of COVID-19, or had health safety concerns.
An operational manual was developed to ensure the fieldwork quality. A centralised data management system, progress reports, and field supervision continuously monitored the fieldwork. Approximately 10% of interviews were rechecked within one week. Quality measures also included checking on coding and ensuring 100% item response.
Wave 3 included 1006 respondents; 56.7% were recontacted respondents, a lower percentage than in Wave 2 (71.3%). 27.2% of the recontacted respondents (155/570) and 86.5% of the new participants (377/436) were interviewed face-to-face. Figure 1 shows the details of the follow-up and retention rates by wave.
CommentsThe 2021 EUREST-PLUS Spain Survey provides a unique opportunity to assess tobacco control policies and changes in smoking behaviour in Spain. The prospective longitudinal design allows the assessment of multiple domains over time, and repeated cross-sectional analyses facilitate multiple assessments in a representative sample of smokers. The COVID-19 questions will provide important insights into the pandemic impact on smoking behaviour and related psychological beliefs.
Several cross-sectional and longitudinal analyses are planned, including:
- •
Determinants of product use and product transitions.
- •
Factors predicting intention to quit.
- •
Correlates of receiving smoking cessation advice from health professionals.
- •
Changes in smoking behaviour during the COVID-19 pandemic.
- •
Incidence of COVID-19 among smokers.
- •
Support for exceptional smoking measures during the lockdown.
- •
Support for smoke-free policies in indoor/outdoor private/public places.
These studies will assess several outcomes and their interaction with smoking characteristics (e.g., nicotine dependence, cigarette frequency, quit attempts) and other psychological and contextual factors (e.g., smoking cessation counselling, knowledge of health effects, psychological beliefs about smoking, harm perception), as well as their association with specific policies (e.g., tobacco advertising, health warnings, restrictions on tobacco sales). Other multi-country analyses using the 2021 Spanish data will include the reasons for using electronic cigarettes, knowledge of the harm caused by tobacco, and beliefs about the harmfulness of novel tobacco products.
The longitudinal design of the ITC EUREST-PLUS Spain Survey constitutes both its main strength and limitation. Despite losses to follow-up, the complex design provides valid and representative information of adult smokers in Spain. Nevertheless, further publications will assess this potential bias by comparing sociodemographic and smoking characteristics between participants and non-participants. Although information from questionnaires is susceptible to information bias, the ITC survey is a validated instrument used in 31 countries. We offered in-person and telephone interviews to maximise participation during the pandemic, but this may have influenced responses; further analyses will be adjusted for the interview type. The survey design ensures data quality with strong potential for analysis of intra- and inter-country differences. Researchers, clinicians, and policymakers will benefit from the latest information on smoking behaviour in Spain, contributing to the evidence base on the effectiveness of current tobacco control policies.
Further, these data will enable empirical studies of social inequalities in tobacco use through analyses of behaviour, knowledge, and beliefs, and policy effectiveness through differences in key equity variables. Understanding these differences is essential for targeting interventions to vulnerable groups.
Availability of databases and material for replicationData available upon request (ITC Project: Dr. Geoffrey T Fong; e-mail: gfong@uwaterloo.ca; Catalan Institute of Oncology: Dr. Esteve Fernández; e-mail: efernandez@iconcologia.net).
Protocol registryThis protocol has not been registered.
Editor in chargeCarlos Álvarez-Dardet.
Authorship contributionsE. Fernández, G.T. Fong and C.I. Vardavas contributed to designing the ITC EUREST-PLUS 6E Surveys, the previous versions of the current survey. E. Fernández, G.T. Fong, M. Fu and C.I. Vardavas contributed to designing the ITC EUREST-PLUS Spain Survey and preparing the study protocol. Y. Castellano, E. Fernández, M. Fu and O. Tigova are the Spanish team responsible for the initial idea to conduct an additional follow-up in Spain and preparing the research proposal for the national grant call. All authors revised the successive versions of the study protocol and the questionnaire until achieving the final versions. M. Fu, S.C. Kaai and A.C.K. Quah coordinated data collection and supervised the fieldwork. P. Driezen and M.E. Thompson supervised the quality of data, prepared the datasets, and created the weights that allow conducting cross-sectional and longitudinal analyses using data from this research. Y. Castellano, E. Fernández, M. Fu and O. Tigova conceptualized the current report, and statistics were conducted by Y. Castellano, with the support of P. Driezen and M.E. Thompson. Y. Castellano, E. Fernández, M. Fu and O. Tigova interpreted the data and planned how to show the results. M. Fu wrote the first draft of the manuscript, with the support of Y. Castellano, E. Fernández and O. Tigova, and all authors substantially contributed to its successive versions. All authors approved the final version. E. Fernández is the guarantor.
Conflicts of interestThe research teams want to thank Matthew Grey, data analyst from the University of Waterloo, for his important contribution in checking and verifying the data of the ITC EUREST-PLUS Spain Survey. The authors at ICO-IDIBELL thank CERCA Programme from the Generalitat de Catalunya, for its institutional support to IDIBELL.
The EUREST PLUS Spain Project is partially funded by the Instituto de Salud Carlos III (grant PI17/01338, co-funded by European Regional Development Fund ERDF, a way to build Europe) and the Canadian Institutes of Health Research (Foundation grant FDN-148477). Y. Castellano, E. Fernández, M. Fu, and O. Tigova are partly supported by the Ministry of Universities and Research, Government of Catalonia (2021SGR00906). Additional support is provided by the Canadian Institutes of Health Research (Foundation grant FDN-148477) to A.C.K. Quah, P. Driezen, M.E. Thompson, S.C. Kaai and G.T. Fong for the work on this manuscript. Additional support to G.T. Fong is also provided by a Senior Investigator Grant from the Ontario Institute for Cancer Research.
G.T. Fong has been an expert consultant for the Government of Singapore to review scientific evidence on standardised packaging and an expert witness or consultant for governments defending their country's policies or regulations in litigation. All other authors have no conflicts of interest to declare.