To estimate the seroprevalence of SARS-CoV-2 antibodies in the Valencian Community (Spain) in October 2022, when BA.5 was the predominant variant.
MethodCross-sectional, region-wide, population-based serosurvey study in 88 randomly selected primary care centers of the Valencian Community.
ResultsSeroprevalence of anti-nucleocapsid (indicative of past infection) and total receptor binding domain (indicative of past infection or vaccination) antibodies was 71.0% (confidence interval [CI]: 67.8-74.2) and 98.4% (CI: 97.5-99.3), respectively. 66.7% (CI: 63.4-70.0) of the population shows hybrid immunity, but only 43.2% in those 80 and over.
ConclusionsThe high proportion of hybrid immunity detected is relevant for public health strategies. A second vaccination booster was advisable in the elderly population.
Estimar la seroprevalencia de anticuerpos frente al SARS-CoV-2 en la Comunidad Valenciana (España) en octubre de 2022, cuando BA.5 era la variante predominante.
MétodoEstudio transversal de base poblacional de ámbito autonómico en 88 centros de atención primaria de la Comunidad Valenciana seleccionados aleatoriamente.
ResultadosLa seroprevalencia de anticuerpos antinucleocápside (indicativos de infección previa) y frente al dominio de la unión al receptor (indicativos de infección o vacunación) fue del 71,0% (intervalo de confianza [IC]: 67,8-74,2) y del 98,4% (IC: 97,5-99,3), respectivamente. El 66,7% (IC: 63,4-70,0) de la población mostraba inmunidad híbrida, pero solo el 43,2% de los mayores de 80 años.
ConclusionesLa alta proporción de inmunidad híbrida detectada era relevante para las estrategias de salud pública, pero era aconsejable un segundo refuerzo de vacunación en la población anciana.
Whereas individual use of antibody (Ab) tests has shown very limited value during the Covid-19 pandemic, population serological surveys have proven usefulness to characterize relevant epidemiological parameters and to guide public health strategies against COVID-19.1 As of October 2022, in the Valencian Community, Spain, with around five million inhabitants, 94% of the population aged 12 and over was fully vaccinated, and 58% had a booster vaccination administered (about 95% in the over 60s).2 Until that month, the Valencian Community had reported around 1.6 million SARS-CoV-2 infection cases (almost a third of the population),3 yet the cumulative incidence of infection may be underestimated due to the large proportion of asymptomatic people and the reduction in testing after the emergence of Omicron. A population serosurvey in April 2022 showed that at least 52% of the Valencian Community population had been infected.4
Few serosurvey studies involving the general population and extending into the Omicron BA.5 wave have been published.5,6 As a part of the ProVaVac Valencian COVID-19 vaccine research program launched by the Valencian Community Government in March 2021, we conducted a population-based study aimed primarily at estimating the seroprevalence of SARS-CoV-2 Ab in the general Valencian Community population in October 2022, when BA.5 was the predominant variant in Spain.
MethodsDesign and settingCross-sectional, region-wide, population-based study using residual blood samples, conducted in randomly selected primary care centers (PCC) of the Valencian Health System in October 2022. The Valencian Health System, part of the Spanish National Health System,7 is an extensive network of public hospitals, more than 200 PCC and public health structures funded and mostly provided by the Valencian Government, which covers about 97% of the Valencian Community population. The Valencian Health System information system use a single personal identification number allowing the link of several publicly owned population-based healthcare integrated databases8 with comprehensive clinical, laboratory and administrative information for the Valencian Community inhabitants, including the Valencian Microbiology (MiVa) Network, a microbiology registry from all public Valencian Community laboratories with data of COVID-19 Active Infection Diagnostic Tests [AIDT] from all public and private laboratories (including antigen rapid test carried out at PCC, accident & emergency departments or pharmacies, but not those performed at home) and the population vaccination registry, which includes all vaccinations administered in the Valencian Community and data from residents vaccinated in other regions.
Sample obtentionThe sample was randomly selected and stratified proportionally to the age-sex distribution of the Valencian Community population (oversampling the age 0-9 years and 80 and over groups and reducing of the 35–49 and 50–64 years groups) and distributed in 88 PCC (randomized, among those 202 PCC with more than 7000 assigned people, to simplify logistics) according to the size of the population served in each one of them. Sample size was determined at 1127 participants (around±2% precision assuming a prevalence of anti-N Ab of 75% and a confidence level of 90%). Samples were obtained from an additional 5ml tube collected from patients having blood drawn on doctor's orders for any reason and meeting the age/sex selection criteria assigned to each PCC. A total of 787 samples were received (response rate: 69.8%). Two samples could not be linked to patient data, leaving 785 samples for statistical analysis. 66% of the losses corresponded to people under 20 years old (not enough youngers with laboratory tests ordered on the day of sampling at the selected PCC). Other losses were due to local holidays or failure to collect lab samples by the PCC on the assigned day.
EthicsRequirement for informed consent was waived by the Valencian Research Ethics Committee of Public Health (ref. 20220408/02) because the project was developed under the epidemiological surveillance competences of the Valencian Community Department of Health.
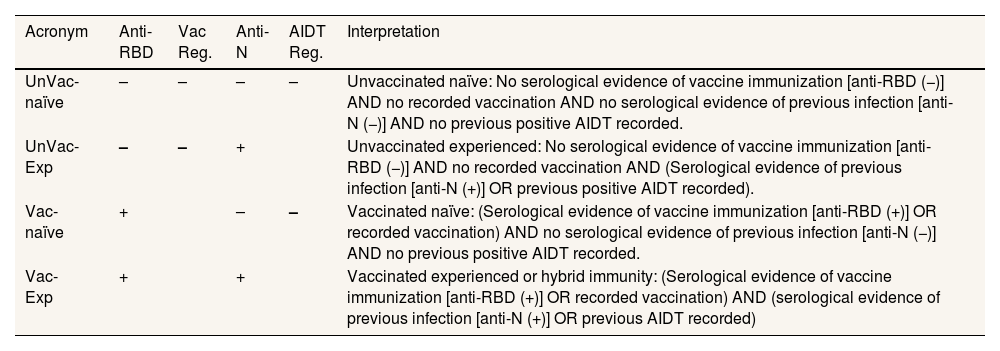
AnalysisAccording to vaccination status from the vaccination registry, SARS-CoV-2 infection recorded before recruitment, and the results of SARS-CoV-2 Ab assays, participants were grouped into four categories (Table 1): vaccinated/experienced (Vac-Exp), vaccinated/naïve (Vac-Naïve), unvaccinated/experienced (UnVac-Exp) and unvaccinated/naïve (UnVac-Naïve). SARS-CoV-2-RBD-total antibodies and nucleocapsid (N)-reactive IgG antibodies were measured in sera using the Roche Elecsys® anti-SARS-CoV-2 S and Elecsys® anti-SARS-CoV-2N assays (Roche Diagnostics, Pleasanton, CA, USA), respectively, following the manufacturer's recommendations. Age-sex proportions with 95% confidence intervals (95%CI) were estimated. Since no significant differences were found between men and women (see Tables I and II in online Appendix), results are shown by age strata. Total results were weighted by the proportion of Valencian Community population in each age/sex stratum. The analyses were performed using STATA 17.0 (StataCorp, College Station, Texas, USA).
Classification of participants according to their vaccination status and recorded SARS-CoV-2 infection at the time of recruitment.
| Acronym | Anti-RBD | Vac Reg. | Anti-N | AIDT Reg. | Interpretation |
|---|---|---|---|---|---|
| UnVac-naïve | – | – | – | – | Unvaccinated naïve: No serological evidence of vaccine immunization [anti-RBD (−)] AND no recorded vaccination AND no serological evidence of previous infection [anti-N (−)] AND no previous positive AIDT recorded. |
| UnVac-Exp | – | – | + | Unvaccinated experienced: No serological evidence of vaccine immunization [anti-RBD (−)] AND no recorded vaccination AND (Serological evidence of previous infection [anti-N (+)] OR previous positive AIDT recorded). | |
| Vac-naïve | + | – | – | Vaccinated naïve: (Serological evidence of vaccine immunization [anti-RBD (+)] OR recorded vaccination) AND no serological evidence of previous infection [anti-N (−)] AND no previous positive AIDT recorded. | |
| Vac-Exp | + | + | Vaccinated experienced or hybrid immunity: (Serological evidence of vaccine immunization [anti-RBD (+)] OR recorded vaccination) AND (serological evidence of previous infection [anti-N (+)] OR previous AIDT recorded) | ||
AIDT Reg: Active Infection Diagnostic Test recorded in the MiVa Network Microbiology Register; Anti-N: nucleocapsid antibodies; Anti-RBD: receptor binding domain antibodies; Vac Reg: Vaccination Registry.
Of the weighted sample, 90.4% was vaccinated with at least one dose (see Table III in online Appendix). By age, all groups were above 90% except the 0-9 years old group (those under 6 were not candidates for vaccination). 59.2% of the weighted sample and more than 90% of people over 65 years had at least one booster dose. 30.6% of the weighted sample had a previous positive AIDT result registered on the MiVa Network, of which 8.1% had no detectable anti-N Ab.
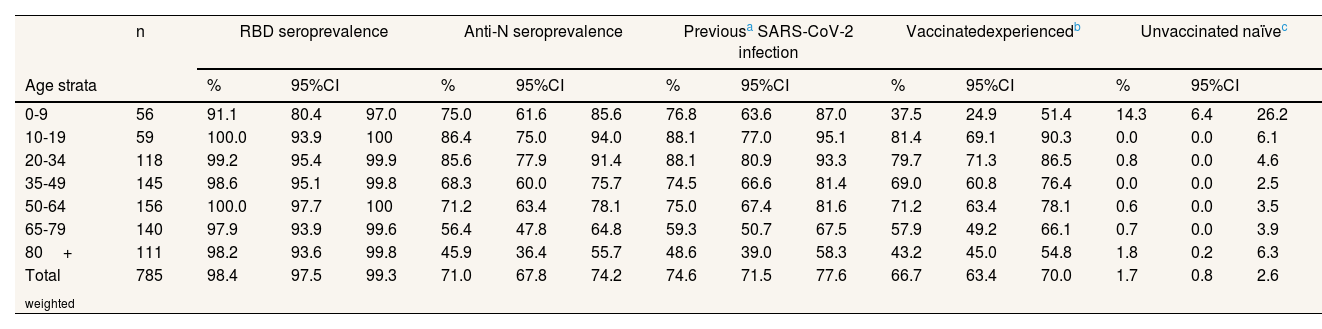
Of the weighted sample, 98.4% maintained detectable RBD Ab against SARS-CoV-2, either due to vaccination or infection (Table 2). Even in the 0-9 age group, the proportion exceeds 90%. Of the weighted sample, 71% maintained detectable anti-N Ab, with higher figures in youngers (>80% between 10 and 34 years) than in those 65 and over (around 50%). When adding patients with at least one positive AIDT registered, 74.6% of the weighted sample had been infected at some point by SARS-CoV-2. 66.7% were Vac-Exp, 23.7% were Vac-Naïve and 7.9% were UnVac-Exp; the remaining 1.7% were UnVac-naïve, mostly people under 10 years.
Serological status and hybrid immunity in the Valencian Community, October 2022.
| n | RBD seroprevalence | Anti-N seroprevalence | Previousa SARS-CoV-2 infection | Vaccinatedexperiencedb | Unvaccinated naïvec | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age strata | % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | % | 95%CI | ||||||
| 0-9 | 56 | 91.1 | 80.4 | 97.0 | 75.0 | 61.6 | 85.6 | 76.8 | 63.6 | 87.0 | 37.5 | 24.9 | 51.4 | 14.3 | 6.4 | 26.2 |
| 10-19 | 59 | 100.0 | 93.9 | 100 | 86.4 | 75.0 | 94.0 | 88.1 | 77.0 | 95.1 | 81.4 | 69.1 | 90.3 | 0.0 | 0.0 | 6.1 |
| 20-34 | 118 | 99.2 | 95.4 | 99.9 | 85.6 | 77.9 | 91.4 | 88.1 | 80.9 | 93.3 | 79.7 | 71.3 | 86.5 | 0.8 | 0.0 | 4.6 |
| 35-49 | 145 | 98.6 | 95.1 | 99.8 | 68.3 | 60.0 | 75.7 | 74.5 | 66.6 | 81.4 | 69.0 | 60.8 | 76.4 | 0.0 | 0.0 | 2.5 |
| 50-64 | 156 | 100.0 | 97.7 | 100 | 71.2 | 63.4 | 78.1 | 75.0 | 67.4 | 81.6 | 71.2 | 63.4 | 78.1 | 0.6 | 0.0 | 3.5 |
| 65-79 | 140 | 97.9 | 93.9 | 99.6 | 56.4 | 47.8 | 64.8 | 59.3 | 50.7 | 67.5 | 57.9 | 49.2 | 66.1 | 0.7 | 0.0 | 3.9 |
| 80+ | 111 | 98.2 | 93.6 | 99.8 | 45.9 | 36.4 | 55.7 | 48.6 | 39.0 | 58.3 | 43.2 | 45.0 | 54.8 | 1.8 | 0.2 | 6.3 |
| Total weighted | 785 | 98.4 | 97.5 | 99.3 | 71.0 | 67.8 | 74.2 | 74.6 | 71.5 | 77.6 | 66.7 | 63.4 | 70.0 | 1.7 | 0.8 | 2.6 |
95%CI: 95% confidence interval.
As of October 2022, after the BA.1, BA.2 and BA.5 waves,3 three out of four people in the Valencian Community had been infected and two out of three had hybrid immunity, although less than one in three had a positive AIDT registered in the MiVa Network. Differences with the serosurvey of April 2022 (anti-N: 47.9%; Vac-exp: 51.9)4 indicate a high transmission during spring and summer 2022, when around 25% of the Valencian Community population became infected (see Table IV in online Appendix). These results are consistent with seroprevalence studies carried out after the BA.2 wave in British Columbia (Canada)5 and Geneva (Switzerland),6 with figures between 61% and 72% for anti-N seroprevalence.
Our study has some limitations. First, people who have been ordered a lab tested may be different from the general population in propensity to infection. Second, we excluded PCC with less than 7000 people assigned, excluding a good part of rural areas, although these usually organize blood drawn in other most populated villages. And third, precision in the pediatric population was markedly reduced by the low number of lab orders in younger groups.
In summary, by October 2022 around three quarters of the Valencian Community population had been infected with SARS-CoV-2 and, due to extensive vaccination, most exhibited hybrid immunity. The high proportion of hybrid immunity is relevant from a public health perspective since these people foreseeably have better and longer lasting protection against the development of severe COVID-19.9,10 The relatively low proportion of hybrid immunity among the elderly advised for a second vaccination booster in people 65 and over.
Availability of databases and material for replicationThe datasets used in this article are not directly available due to Valencia Health Agency Resolution 2009/13312 (available at: http://www.san.gva.es/documents/152919/157920/resolucionsolicituddatos.pdf) which limits the cession of data to third parties without previous authorization from their Data Commission. Requests to access the data sets should be directed to Management Office of the Data Commission in the Valencia Health Department (email: solicitud_datos@gva.es; telephone numbers:+34 961-928207;+34 961-928198). Upon request, authors can get access to the databases to verify the accuracy of the analysis or the reproducibility of the study.
Few serosurvey studies involving the general population and extending into the Omicron BA.5 wave have been published. We conducted a population-based study to estimate the cumulative incidence of SARS-CoV-2 infection in the population of the Valencian Community, Spain.
What does this study add to the literature?In October 2022, three out of four people in the Valencian Community had been infected and two out of three had hybrid immunity. Compared to April 2022, results indicate a high transmission during spring/summer, when around 25% of the population became infected.
What are the implications of the results?The high proportion of hybrid immunity detected is relevant from a public health perspective. The relatively low proportion of hybrid immunity among the elderly advised for a second booster in people 65 and over.
Miguel Ángel Negrín Hernández.
Transparency declarationThe corresponding author on behalf of the other authors guarantee the accuracy, transparency and honesty of the data and information contained in the study, that no relevant information has been omitted and that all discrepancies between authors have been adequately resolved and described.
Authorship contributionsS. Peiró, D. Navarro, H. Vanaclocha and R. Limón conceived and planned the study. A. García-Sempere, S. Peiró and D. Navarro drafted the study protocol (which was revised and approved by the ProVaVac Committee and the General Directorates of Health Care and Public Health of the Valencia Government Health Department). A. García-Sempere, H. Vanaclocha, R. Limón, D. Navarro and S. Peiró, prepared and coordinated the field work. D. Navarro and E. Giménez carried out the serologic and microbiological analyses. E. Giménez and H. Vanaclocha and linked the databases, and A. García-Sempere prepared the anonymized databases for analysis. A. García-Sempere and S. Peiró carried out the statistical analysis. A. García-Sempere, S. Peiró and D. Navarro drafted the first version of the manuscript with inputs from all authors (and several members of the ProVaVac Committee). All authors provided critical feedback and approved the final version submitted.
AcknowledgementsWe are grateful to the staff and nurses of primary care centers participating in this study. We also thank Ana Berenguer, General Director of Analysis and Public Policies of the Presidency of the Valencia Government; Jorge Camacho and Eliseo Albert, from the Microbiology Service of the Valencia Clinic University Hospital; and José Sánchez-Payá, from the Alicante Institute for Health and Biomedical Research (ISABIAL). We also appreciate the contributions of the Valencian Vaccine Research Program (ProVaVac) group, during the preparation of the study protocol, the field work and the preparation of the final report of the study. The ProVaVac group, during the development of this study, was integrated by D.J. Burks (Príncipe Felipe Research Center, CIPF, Valencia, Spain); A. Cervantes, J. Redón and D. Navarro (INCLIVA Health Research Institute, Valencia, Spain); I. Comas (Biomedicine Institute of Valencia, Spanish Research Council, IBV-CSIC, València, Spain); J. Díez-Domingo, F. González-Candelas and S. Peiró (Foundation for the Promotion of Health and Biomedical Research of the Valencian Community, FISABIO, Valencia, Spain); C. Ferrer (Castelló Provincial Hospital Research Foundation, Castelló, Spain); I. Hernández (Miguel Hernandez University, Elx, Spain); R. Meneu (Health Services Research Institute Foundation, Valencia, Spain); N. Oliver (ELLIS Foundation, Alicante, Spain); J. Sánchez-Payá (Alicante Institute for Health and Biomedical Research, ISABIAL, Alicante, Spain); G. Sanz (Health Research Institute La Fe, València, Spain); J.M. Sempere (University of Alicante, Alicante, Spain); H. Vanaclocha (General Directorate of Public Health, Department of Health, Valencia Government, Valencia, Spain); and E. Zapater (Valencia General and University Hospital Research Foundation, Valencia, Spain).
FundingProject funded by the Valencia Government Health Department and the EU Operational Program of the European Regional Development Fund (ERDF) for the Valencian Community 2014-2020, within the framework of the REACT-EU program, as the Union's response to the COVID-19 pandemic. A. García-Sempere was supported for this funding.
Conflicts of interestNone.