The Spanish influenza surveillance system (SISS) maintained its activity during the summer of 2009 to monitor the influenza pandemic.
ObjectivesTo describe pandemic influenza activity from May to September 2009 and to estimate the effectiveness of the 2008-9 seasonal influenza vaccine against laboratory-confirmed pandemic (H1N1) 2009 influenza.
MethodsData from the SISS were used to identify the trend of pandemic (H1N1) 2009 influenza outside the influenza season. For the effectiveness study, we compared the vaccination status of notified cases [influenza-like illnesses (ILI) laboratory confirmed as pandemic influenza] with that of the test-negative controls.
ResultsThe first laboratory-confirmed case of the pandemic virus was notified in the system in week 20/2009. The ILI rate increased gradually in the study period, exceeding basic activity in week 38. The proportion of pandemic (H1N1) 2009 influenza viruses detected by the system represented 14% in week 20/2009 and rapidly increased to 90% in week 34. The adjusted vaccine effectiveness of the 2008-9 seasonal vaccine against laboratory-confirmed pandemic influenza was 12% (-30; 41).
ConclusionsThe SISS became an essential tool for pandemic monitoring in Spain. The improved SISS will provide more accurate information on influenza activity in future seasonal or pandemic waves. Using surveillance data, we could not demonstrate the effectiveness of the seasonal 2008-9 vaccine against laboratory-confirmed pandemic influenza.
El Sistema de Vigilancia de Gripe en España (SVGE) continuó y reforzó su actividad durante el verano de 2009 con el objetivo de vigilar la evolución de la pandemia en España.
ObjetivosDescribir la actividad de la gripe pandémica en España de mayo a septiembre de 2009 y estimar la efectividad de la vacuna antigripal estacional 2008-2009 frente a casos confirmados de gripe pandémica (H1N1) 2009.
MétodosSe utilizaron datos del SVGE para presentar la evolución de la pandemia por virus (H1N1) 2009 fuera de la temporada de vigilancia 2008-2009. Para el estudio de la efectividad vacunal se comparó el estado vacunal de los casos de gripe pandémica confirmados por laboratorio con el de los casos negativos para el virus de la gripe (controles negativos).
ResultadosEl primer caso confirmado de virus pandémico se notificó en la semana 20/2009. La incidencia de gripe aumentó paulatinamente durante el periodo estudiado y sobrepasó el umbral basal en la semana 38/2009. La proporción de virus (H1N1) 2009 detectada por el SVGE fue del 14% en la semana 20 y aumentó rápidamente, llegando a alcanzar el 90% en la semana 34. La efectividad ajustada de la vacuna antigripal 2008-2009 frente a casos confirmados de gripe pandémica fue del 12% (-30; 41).
ConclusionesEl SVGE se adaptó y mejoró de forma rápida a las exigencias nacionales e internacionales de vigilancia de la pandemia. Esta mejora supone información más precisa y de calidad en futuras ondas epidémicas/pandémicas. Con los datos obtenidos en vigilancia no se pudo demostrar alguna efectividad de la vacuna antigripal 2008-2009 frente a los casos de gripe pandémica confirmados por laboratorio.
On 27 April 2009, Spain reported the first case of the influenza pandemic (H1N1) 2009 infection in Europe.1 Since then, the influenza surveillance strategy was adapted to the pandemic progress, according to the recommendations of the Spanish pandemic preparedness plan for the containment and mitigation phases.2,3 Following WHO alert of pandemic phase 5,4 the European Centre for Disease Prevention and Control (ECDC) recommended to the European Union Member States to continue the influenza surveillance within the influenza surveillance sentinel systems.5 After pandemic phase 6 declaration,6 a gradual transition from case-based reporting to sentinel surveillance and surveillance of severe cases was implemented in Spain.3
The Spanish Influenza Surveillance System (SISS) was established in 19967 and since then it was integrated in European Influenza Surveillance Network (former European Influenza Surveillance Scheme).8 The objectives of the surveillance system are to provide timely epidemiological and virological information on influenza activity in Spain from week 40 of one year to week 20 of the following year. In line with ECDC recommendation, SISS maintained its activity during the summer 2009, from week 20/2009 to week 39/2009. The sixteen Spanish regional sentinel networks and regional laboratories integrated in the surveillance system were recommended to: 1) increase the number of sentinel general practitioners (GPs) and paediatricians (PDs) participating in the network; 2) swab all patients meeting the influenza case definition and consulting a sentinel GP; and 3) train sentinel GPs and PDs for adopting the influenza European Commission case definition.9
Influenza vaccine effectiveness of the trivalent seasonal vaccine against the pandemic (H1N1) 2009 virus has been estimated in other countries10–12 using different designs. The routine surveillance system was used in Australia to estimate the effectiveness of trivalent vaccine during the influenza season in the Southern hemisphere, comparing the vaccination status of the laboratory confirmed cases with that of the test-negative controls.10 The test-negative design was previously used to estimate trivalent seasonal influenza effectiveness13,14 and pilot tested using the SISS15–17 as part of the ECDC funded I-MOVE project.18
The aim of this paper is to describe the epidemiological and virological activity of the influenza pandemic (H1N1) 2009 in Spain, from May to September 2009, using the information obtained from the influenza surveillance. We also aimed at measuring the effectiveness of the trivalent seasonal vaccine 2008-9 against medically attended laboratory confirmed pandemic influenza (H1N1) 2009 in order to guide public health recommendations.
MethodsDescription of Spanish Influenza Surveillance SystemThe Spanish influenza surveillance system comprises networks of sentinel physicians and network-affiliated laboratories, including the National Influenza Centre-Madrid (NIC-Madrid, WHO National Influenza Centre, National Centre of Microbiology). In addition to the specimens taken by sentinel physicians, regional laboratories also collect specimens from non-sentinel sources (i.e. hospitals, collaborating laboratories). Influenza detections from non-sentinel sources are notified to the system. In the season 2008-9, SISS included over 524 GPs and 173 PDs from 16 Spanish Autonomous Communities (ACs). The system covered 926,092 inhabitants, representing 2.07% of the total population of the 16 ACs. GPs and PDs report on weekly basis cases of influenza like illness (ILI) detected in their reference populations. In the 2008-9 season, 12 Spanish sentinel networks used the ILI definition of International Classification of Health Problems in Primary Care19: 1) context of influenza epidemic and four of the criteria listed in the point 2; or 2) six of the following criteria: onset within 12hours, cough, fever, chills, prostration and weakness, myalgia or general pain, rhinitis, pharyngitis, contact with a case. Four Spanish sentinel networks used an ILI definition based on the European case definition9: sudden onset of symptoms, and at least one out of four systemic symptoms: fever or feverishness, malaise, headache, myalgia; and at least one out of three respiratory symptoms: cough, sore throat, shortness of breath; and in the absence of other suspected clinical diagnosis.
For virological influenza surveillance, sentinel physicians took nasal or nasopharyngeal swabs, and sent them for influenza virus detection to the regional laboratories and network affiliated laboratories in each Autonomous Communities. Starting the influenza enhanced surveillance, all patients meeting the ILI case definition were swabbed. The swabbing strategy changed along the surveillance period, from all cases to a systematic swabbing, according to network resources and laboratory capacity. If the assay to confirm the influenza pandemic (H1N1) 2009 virus was not available at the regional laboratories, specimens were sent to the the National Reference Laboratory. Two independent assays were used for detection of pandemic influenza (H1N1) 2009 virus: 1) a multiplex reverse transcription (RT)-nested PCR assay for generic detection of Influenza A, B and C designed in the Nucleoprotein gene, and 2) a multiplex RT-nested PCR assay for sub-typing of the Haemagglutinin gene of influenza A viruses. Amplified products from both RT-PCR assays were sequenced and identification of (H1N1) 2009 virus was performed after sequence analysis.20 For molecular characterizations of resistance to antivirals, analysis of Matrix and Neuraminidase genes were also performed in all available isolates.
The data collected in the SISS included: sex, age, sentinel/non sentinel source, date of symptoms onset, date of swabbing, vaccination status, influenza laboratory confirmation, influenza type/subtype and influenza strain. Data was entered weekly, by each regional sentinel network in a web-based application and analysed at central level by the National Centre of Epidemiology, to provide timely information on the evolution of influenza activity at ACs and national level (http://vgripe.isciii.es/gripe).
Study of influenza vaccine 2008-9 effectivenessBased on SISS data, we conducted a case-control study using the laboratory results of swabs taken from week 20 to week 39 (May-September 2009). Cases were medically attended ILI, laboratory confirmed for pandemic influenza (H1N1) 2009. Controls were medically attended ILI that tested negative for any influenza type in a SISS-affiliated laboratory (test-negative controls).
The WHO recommended influenza triple vaccine for Northern hemisphere in the 2008-9 season21 was the exposure for the effectiveness study. Annual vaccination is recommended in Spain for the high risk groups for influenza complications: patients with chronic conditions at any age, health care workers, elderly over 65 years old (over 60 years in some ACs).22 Vaccination is conducted at the GP/PD level during the annual campaign that lasted from October to December 2008, depending on Autonomous Community organization. Data on vaccination is collected by the GP/PD according to the patient clinical history. We excluded from the analysis ILI cases that had unknown vaccination status, no age nor laboratory results reported in the system.
For statistical analysis, we compared the characteristics of cases and controls by sex and age using Pearson's chi-square test. We calculated the crude and adjusted odds ratios (OR), their corresponding 95% confidence intervals (95%CI) using logistic regression and computed the vaccine effectiveness as (1-OR)*100. Variables included in the regression model were: age group, sex, month of swabbing and Autonomous Community. We adjusted for month of swabbing to control for differences in influenza incidence along the period of the study. After checking for clustering by Autonomous Community, we used a two level logistic regression model, introducing AC as random effect.23 We used STATA/IC 10 to carry out the statistical analysis.
ResultsPandemic influenza (H1N1)2009 activity, May-October 2009, SpainThe Influenza 2008-9 season (from week 40/2008 to week 19/2009) was moderate, associated to a mixed circulation of influenza A(H3N2) during the epidemic wave, and a further predominant circulation of B virus.24 The number of sentinel physicians participating in SISS increased from 525 GPs and 173 PDs in week 20/2009 to 647 GPs and 220 PDs in week 39/2009. The population under surveillance increased with 22%, up to 2.53% in week 39/2009.
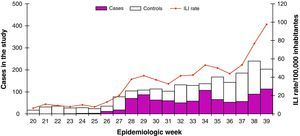
From week 20/2009 to week 25/2009 (first weeks of the extended surveillance period), the influenza activity was low (ILI rate below 12 cases/100,000). The ILI rate started to increase steadily from week 26 (28 June – 4 July 2009), although influenza incidence rates maintained below baseline levels, overpassing it in week 38 (19-25 September 2009). Figure 1 shows the evolution of weekly ILI rates during 2008-9 season, during the usual surveillance period (week 40/2008 to week 20/2009) and during the extended surveillance period (May-September 2009).
From May to September 2009, the proportion of influenza sentinel positive specimens increased from 0 to 31%. From week 28, this proportion was higher than 30% (fig. 1).
The highest ILI rate was in the age group 5-14 years old (233 cases/100,000 population), followed by the 0-4 age group (153 cases/100,000 population) in week 39/2009.
The first influenza pandemic (H1N1) 2009 virus was detected in week 20, from a non-sentinel source. In week 23/2009 the sentinel system detected the first (H1N1) 2009 confirmed case. Among the total influenza virus detected by the sentinel and non-sentinel sources, the proportion of pandemic influenza (H1N1) 2009 was 14% in week 20/2009, 67% in week 21/2009 and more than 90% from week 34/2009.
In the study period, 3245 sentinel ILI cases were swabbed (3220 cases with age reported) representing 70% of total ILI medically attended by sentinel GPs/PDs, ranging from 85% in 0-4 aged group to 66% in over 64 years old (table 1). Out of 919 sentinel confirmed influenza, 92% were pandemic influenza (H1N1) 2009 (table 1).
Number of sentinel influenza-like illnesses tested, proportion of swabbed ILI, proportion of pandemic influenza and vaccinated cases and controls and their corresponding odds ratio (OR) by age group, May-September 2009, Spain.
| Age group | ILI cases n (%) | Swabbed ILI n (%) | Influenza A(H1N1)2009/All influenza viruses n (%) | Influenza A(H1N1)2009/Influenza A subtyped n (%) | Vaccinated n (%) | Cases Vaccinated/All (%) | Controls Vaccinated/All (%) | OR (95%CIb) |
| 0-4 years | 546 (12.0) | 464 (85.0) | 47/55 (85.4) | 47/47 (100.0) | 24/388 (6.19) | 3/47 (6.38) | 21/341 (6.16) | 1.04 (0.19; 3.69) |
| 5-14 years | 1120 (24.5) | 839 (75.0) | 328/345 (95.1) | 328/329 (99.7) | 39/733 (5.32) | 18/328 (5.49) | 21/405 (5.19) | 1.06 (0.52; 2.13) |
| 15-44 years | 2174 (47.6) | 1443 (66.4) | 389/429 (91.7) | 389/394 (98.7) | 43/1183 (3.63) | 11/389 (2.83) | 32/797 (4.02) | 0.69 (0.31; 1.44) |
| 45-64 years | 561 (12.3) | 365 (65.1) | 74/83 (89.1) | 74 /77 (98.7) | 46/309 (14.9) | 9/74 (12.2) | 37/235 (15.7) | 0.74 (0.29; 1.67) |
| +64 years | 166 (3.6) | 109 (65.7) | 6/7 (85.7) | 6/6 (100.0) | 44/91 (48.4) | 1/6 (16.7) | 43/85 (50.6) | 0.19 (0.00; 1.88) |
| Total | 4567 (100.0) | 3220a (70.5) | 844/919 (91.8) | 844/853 (98.9) | 196/2707 (7.24) | 42/844 (4.98) | 154/1863 (8.27) | |
ILI: influenza like illness.
Molecular characterization of the pandemic influenza viruses detected by RT-PCR assays and subsenquentely sequenced, showed that the circulating viruses were similar to A/California/07/2009(H1N1) strain in both Nucleoprotein and Haemagglutinine genes. Moreover, all pandemic viruses tested were resistant to M2 inhibitors (Adamantanes) and none of them showed mutation in the NA gene which conferred resistance to neuraminidase inhibitors (oseltamivir).
Study of the effectiveness of influenza vaccine 2008-9After the exclusion of influenza types B and C (n=24), no subtyped influenza A cases (n=53), ILI patients with unknown vaccination status (n=148) or no age reported (n=25), we included in the seasonal vaccine effectiveness study 2707 sentinel reported ILI patients: 844 influenza pandemic (H1N1) 2009 cases and 1863 test-negative ILI controls. The weekly number of pandemic (H1N1) 2009 cases and test-negative controls increased by week of swabbing (fig. 2).
The cases were significantly younger than controls (p<0.001): mean age of cases: 20.8 years (standard deviation: 14.4), compared with controls mean age: 25.1 years (standard deviation: 20.1). No difference regarding gender was registered between cases and controls (p=0.202).
A total of 196 (7.24%) ILI patients included in the study were vaccinated with the 2008-9 season trivalent vaccine: 42 (4.98%) cases, and 154 (8.27%) controls. The crude OR was 0.58 (95%CI: 0.39; 0.83). Stratifying by age group (table 1), we found no difference between strata (homogeneity test p=.61) and a Mantel Haenzel combined OR of 0.79 (IC 95%: 0.54; 1.16). The OR adjusted for sex, age group, mounth of swabbing and Autonomous Community, was 0.88 (95%CI: 0.59; 1.30), resulting in an IVE of 12% (95% CI: -30; 41).
DiscussionThe Spanish influenza surveillance system proved its value in an emergency situation, playing a critical role in monitoring the influenza pandemic (H1N1) 2009 in Spain. Sentinel surveillance systems confirmed to be a useful tool to monitor pandemic influenza in different countries and regions.10,25,26
For the first time in 2009, Spain experienced a substantial influenza activity during the summer, due to influenza pandemic (H1N1) 2009 virus. Starting the end of June 2009, the proportion of positive sentinel ILI cases and the proportion of pandemic (H1N1) 2009 viruses among all circulating influenza strains showed a sustained circulation of influenza pandemic (H1N1) 2009 virus in all Spanish ACs and in all age groups. In previous years, virological surveillance conducted out of the usual influenza surveillance season, showed almost no influenza viral circulation.27
Following the surveillance recommendations, the proportion of ILI patients swabbed in the sentinel system increased over 50% comparing with the 2008-9 season.28 In order to cope with the amount of specimens collected, the swabbing strategy changed along the surveillance period (from all cases presenting to sentinel physicians to a systematic swabbing, according to network resources and laboratory capacity). The proportion of patients tested was similar in all age groups showing that during summer 2009, swabbing was not influenced by the patient age as in previous influenza seasons,7 and reflected the distribution of influenza vaccination coverage in Spain by age group.29
Similarities between pandemic (H1N1) 2009 virus and the seasonal H1N1 one,30 might suggest that some effectiveness of the seasonal vaccine 2008-9 which included a H1N1-like antigen for the pandemic strain could be possible. A study in Australia did not show any effect of the Southern hemisphere seasonal vaccine 2009 against medically attended laboratory confirmed pandemic influenza.10 In USA, the results of a study11 using the screening method suggested no effectiveness of the trivalent seasonal vaccine 2008-9 against laboratory confirmed pandemic influenza. In a hospital case-controls study,12 the IVE of the seasonal vaccine 2008-9 against hospitalized pandemic influenza was estimated to be 73% (95%CI: 34-89). However, it was argued that the reported protection might be due to a combination of selection bias and recall bias, as the control group used might not represent the population given rise to cases.31
Our results suggested that the seasonal vaccination 2008-9 did not have any effect against the medically attended laboratory confirmed influenza pandemic (H1N1) 2009. The age groups most affected by the influenza pandemic are not targeted by the seasonal vaccination resulting in a low number of vaccinated in our study population. In addition, we registered an important number of missings in the variable of exposure. Since no information on other confounder factors known to influence the influenza vaccine effectiveness (chronic diseases, functional status, smoking, etc) was collected in the routine surveillance system, we could only adjust for sex, age, mounth of swabbing and autonomous community. These limitations might influence the results of the influenza vaccine effectiveness in both ways.
Controlling for the random effect of Autonomous Community improved our model, even though the vaccine effectiveness point estimate or confidence interval did not change significantly (data not shown). This can be explained by the differences in the vaccination strategies, sentinel GP coverage and health seeking behaviour among the Autonomous Communities.
In conclusion, the Spanish influenza surveillance system was able to rapidly adapt to the national and international requirements for the surveillance of pandemic influenza (H1N1) 2009, becoming an essential tool for pandemic monitoring in Spain. In order to face this challenge, the number of sentinel physicians and population covered by the system increased. In addition, a systematic swabbing of ILI patients was introduced. These improvements in the influenza surveillance system, if sustained, will help to better monitor and provide more accurate information on the influenza activity in the future seasonal or pandemic waves.
Based on the influenza surveillance system, it was possible to estimate the effectiveness of the seasonal vaccine 2008-9 against pandemic influenza (H1N1) 2009, showing no effect. Strengthening the routine data collection by including data on the main confounding factors will provide more accurate estimates of influenza vaccine effectiveness. Since no effect of the seasonal vaccine was expected, vaccination with monovalent pandemic vaccine was recommended in Spain to prevent the pandemic influenza related outcomes.32 The seasonal vaccination campaign started in September 2009 and the pandemic vaccination campaign in November 2009. In the next seasons, the surveillance system will continue to be the basis to monitor influenza vaccine effectiveness.
Authorship contributionThe SISS collected data for this study. A. Larrauri and C. Savulescu equally contributed to the study design and the first draft of the article. A. Larrauri and S. Jiménez analysed the epidemiological and virological data regarding influenza activity. C. Savulescu and S. de Mateo conducted the analysis of the vaccine effectiveness study. P. Pérez Breña, I. Casas, F. Pozo and J. Ledesma confirmed the influenza virus A (H1N1) 2009 pandemic infection in the beginning of the pandemic and carried out the genetic characterization. All authors brought ideas, interpreted the results and revised the drafts. All authors approved the final draft. A. Larrauri is responsible for this article.
Conflict of interestThe authors state that they have no conflicts of interest.
We would like to acknowledge the contribution of the Coordinating Centre for Health Alerts and Emergencies within Spanish Ministry of Health and Social Policy, which has been leading the pandemic influenza control from the early stage of the pandemic and the Spanish Surveillance Sub-Committee of National Plan Preparedness and Response to Pandemic Influenza, both groups being in charge of adapting of the surveillance strategy according to the pandemic progress.
We are thankful to Teresa López for her comments on the analysis for the vaccine effectiveness study and Marta Valenciano for her comments on the final draft of this paper.
SISS: Physicians of the influenza sentinel surveillance networks of: Andalucía, Aragón, Asturias, Baleares, Canarias, Cantabria, Castilla-La Mancha, Castilla y León, Cataluña, Comunidad Valenciana, Extremadura, Madrid, Navarra, País Vasco, La Rioja and Ceuta. Epidemiologists members of the SISS belonging to: Servicio de Vigilancia Epidemiológica y Evaluación, Consejería de Salud, Junta de Andalucía; Servicio de Vigilancia en Salud Pública, Dirección General de Salud Pública, Aragón; Dirección General de Salud Pública y Planificación, Consejeria de Salud y Servicios Sanitarios, Asturias; Servicio de Epidemiología, Dirección General de Salut Pública, Baleares; Servicio de Epidemiología y Prevención, Consejería de Sanidad de Canarias; Sección de Epidemiología, Consejería de Sanidad, Trabajo y Servicios Sociales de Cantabria; Servicio de Epidemiología, Consejería de Sanidad de Castilla-La Mancha; Dirección General de Salud Pública e Investigación, Desarrollo e Innovación, Consejería de Sanidad de Castilla y León; Servicio de Vigilancia Epidemiológica, DGSP, Departament de Salut, Generalitat de Catalunya; Àrea d’Epidemiologia, Conselleria de Sanitat, Comunitat Valenciana; Servicio de Epidemiología, Consejería de Bienestar Social, Junta de Extremadura; Dirección Xeral Saúde Pública de Galicia; Dirección General de Atención Primaria de la Comunidad de Madrid; Servicio de Epidemiología, Consejería de Sanidad de la Región de Murcia; Sección de Vigilancia de Enfermedades Transmisibles, Instituto de Salud Pública de Navarra; Servicio de Vigilancia Epidemiológica, Consejería de Sanidad del País Vasco; Servicio de Epidemiología, Subdirección de Salud Pública de La Rioja; Sección de Vigilancia Epidemiológica, Consejería de Sanidad y Bienestar Social de Ceuta; Centro Nacional de Epidemiología, ISCIII, Madrid. Virologist members of the SISS belonging to laboratories: National Influenza Reference Laboratory, WHO Influenza colaborating Centre (Centro Nacional de Microbiología, ISCIII, Madrid); WHO Influenza colaborating Centre, Facultad de Medicina de Valladolid; WHO Influenza collaborating Centre, Hospital Clínico de Barcelona; Hospital Virgen de las Nieves, Granada; Laboratorio del Hospital Miguel Servet, Zaragoza; Laboratorio del Hospital Nª Srª de Covadonga, Oviedo; Laboratorio del Hospital Son Dureta, Palma de Mallorca; Laboratorio del Hospital Dr. Negrín, Las Palmas de Gran Canaria; Laboratorio del Hospital Universitario Marqués de Valdecilla, Santander; Instituto Valenciano de Microbiología, Valencia; Hospital San Pedro de Alcántara, Cáceres; Servicio de Microbiologia del Hospital Universitario Ramon y Cajal, Madird; Laboratorio de la Clínica Universitaria de Navarra, Pamplona; Laboratorio de Microbiología, Hospital Donostia, San Sebastián; Hospital San Pedro de La Rioja, Logroño; Laboratorio de Microbiología del Hospital de INGESA, Ceuta; Laboratorios de Microbioloxía CH de Vigo y de Ourense Hospital Virgen de la Arrixaca, Murcia.