To assess the effectiveness, safety, and cost-effectiveness of genicular artery embolization (GAE) for the treatment of mild or moderate knee osteoarthritis (KO) refractory to standard treatment, and/or severe KO in individuals not eligible for surgery.
MethodWe conducted a systematic review with meta-analysis, supplemented by a cost-analysis, comparing GAE and standard treatment, from the perspective of the Spanish National Health System (NHS) over a one-year time horizon. The health improvement required for GAE to be deemed cost-effective was quantified, considering a willingness-to-pay threshold of 25 000 €/quality-adjusted life year (QALY).
ResultsWe included two randomized controlled trials in our analysis. Pain estimates showed inconsistent results, and no significant effects were observed for overall function, health-related quality of life, or changes in the need for pain management medication. No serious complications or major adverse events were observed. GRADE quality of evidence ranged from moderate to low. No economic evaluations were identified. Our cost-analysis revealed that GAE would result in an incremental cost of € 3432.37 per patient, requiring a health improvement of 0.137 QALY per patient to be deemed a cost-effective technology.
ConclusionsIn summary, based on moderate to low-certainty evidence, it remains inconclusive whether there is any difference between GAE and standard treatment for KO. However, the use of GAE would increase the costs. Larger randomized controlled trials are needed to determine the effects of using GAE for chronic pain secondary to KO and, consequently, to ascertain whether this technology could potentially become cost-effective from the NHS perspective.
Evaluar la efectividad, la seguridad y el coste-efectividad de la embolización de la arteria genicular (EAG) para el tratamiento de la artrosis de rodilla (AR) leve o moderada, refractaria al tratamiento habitual, o grave en personas no candidatas a cirugía.
MétodoSe llevó a cabo una revisión sistemática con metaanálisis y un análisis de costes para comparar la EAG y el tratamiento habitual, desde la perspectiva del Sistema Nacional de Salud (SNS) español, con un horizonte temporal de 1 año. Se estimó la mejora en salud necesaria para que la EAG se considere coste-efectiva, con un umbral de 25.000 €/año de vida ajustado por calidad (AVAC).
ResultadosSe incluyeron dos ensayos controlados aleatorizados. Los resultados en dolor fueron inconsistentes y no se observaron efectos significativos en la función general, la calidad de vida ni la necesidad de medicación para el dolor. No se observaron complicaciones graves ni eventos adversos mayores. La calidad de la evidencia fue de moderada a baja. No se identificaron evaluaciones económicas previas. El coste incremental de la EAG sería de 3.432,37 €/paciente, requiriendo una mejora de 0,137 AVAC/paciente para ser coste-efectiva.
ConclusionesLa evidencia de certeza moderada a baja no permite concluir si hay diferencias entre la EAG y el tratamiento habitual para la AR. Sin embargo, el uso de la EAG incrementaría los costes. Se necesitan ensayos controlados aleatorizados de mayor tamaño para determinar los efectos de la EAG en el dolor crónico secundario a la AR y establecer si podría ser coste-efectiva desde la perspectiva del SNS.
Knee osteoarthritis (KO) is a leading cause of chronic pain and disability worldwide, particularly among women, individuals over 50, and individuals who are overweight or obese,1,2 with an estimated prevalence of 29.3% in the general population.3
Standard treatment for early-stage KO (mild to moderate) includes exercise, postural measures, weight control,1,4 and pharmacotherapy.4–6 Intra-articular injections of hyaluronic acid or platelet-rich plasma have emerged as newer non-surgical treatment options for managing KO.1,7 Joint replacement surgery is reserved for severe cases with intense pain and functional disability.8 However, many patients experience refractory chronic pain or are not surgical candidates. Additionally, some patients may experience complications associated with long-term pharmacotherapy, such as kidney or liver failure, opioid addiction, or local issues arising from injections.9
Recently, genicular artery embolization (GAE) has emerged as a promising minimally invasive procedure for managing secondary pain to locomotor inflammatory diseases.10 GAE selectively embolizes genicular branches to painful or abnormal vascularized areas,11 typically using microspheres or polyvinyl alcohol particles.12
GAE has been proposed as an alternative or complementary treatment to the standard non-surgical treatment for KO,4–7,13–16 particularly for patients resistant to conventional therapies, including those who cannot or prefer not to undergo surgery. While some studies suggest that GAE may benefit patients across the spectrum of KO severity,13 others suggest its potential for early and low-grade KO, especially in the short and medium term.14 Additionally, this procedure is considered safe, without major complications,4,6,13 although minor complications are not infrequent.13
This study evaluates the effectiveness, safety and cost-effectiveness of GAE in treating mild to moderate KO refractory to standard treatment or severe KO in non-surgical candidates, due to the still existing uncertainty.
MethodSystematic review on effectiveness, safety and cost-effectivenessA systematic review on the clinical effectiveness and safety of GAE for treating mild to moderate KO refractory to standard treatment or severe KO in non-surgical candidates was conducted according to the Cochrane Collaboration methodology,17 with reporting in accordance with the PRISMA statement.18 The protocol was registered in OSF [OSF osf.io/ytxvu]. Complementary, a systematic review of cost-effectiveness were performed following the Campbell-Cochrane Economic Methods.19
- 1)
Information sources and search strategy
The systematic review searches and subsequent identification of their included randomized controlled trials or non-randomized comparative studies was conducted using technological enablers from the Epistemonikos database, in April 2023. The results were automatically incorporated into the Epistemonikos L·OVE platform,20 where subsequent selection was conducted. An alert service for randomized controlled trials and non-randomized comparative studies was also created, which remained active until May 31, 2024.
Additionally, to identify randomized controlled trials or other comparative studies not included in available systematic reviews, searches were conducted in MEDLINE (Ovid), Embase (Elsevier), CENTRAL (Wiley), and CINAHL (EBSCOhost), in October 2023.
Economic evaluations were identified through searches in MEDLINE, Embase, and Web of Science, in June 2023. The terms from this strategy were combined with a strategy specifically designed by the University of York21 to identify economic evaluations.
All search strategies were limited to studies published in English or Spanish within the last 10 years, as the earliest study on joint embolization for pain management in inflammatory musculoskeletal diseases was published in 2013.10
Retrieved references were managed using Zotero 6.0.2322 and deduplicated using Deduklick,23 followed by manual removal of duplicates and study selection in Microsoft Excel.
Furthermore, the bibliography of included articles was manually examined, and studies citing the selected studies were verified through Google Scholar.
Full search strategies for all database searches are provided in Appendix A in Supplementary data.
- 2)
Selection criteria
Studies were eligible for inclusion if they fulfilled the criteria summarized in Table 1.
Table 1.Selection criteria for studies assessing effectiveness and safety.
Criteria category Inclusion criteria Population Individuals with chronic pain secondary to mild or moderate KO refractory to standard treatment and/or severe KO who are not candidates for surgery Intervention GAE Comparator Standard treatment, usual treatment, conservative management, drug therapy (without GAE) or non-intervention Outcome measure Outcomes of interest included KO pain, overall function, HRQoL, need for pain medication, complications, and adverse events. For economic evaluations, the outcomes were: ICER, costs expressed in monetary units, and benefits expressed in QALY, LYG, monetary units, or any of the outcome measures included in the safety or effectiveness section Type of study High-quality systematic reviews were included, as well as randomized controlled trials. In their absence, non-randomized clinical trials and observational comparative studies were consideredFull economic evaluations (either alongside primary studies or modelling based) were included: cost-benefit analysis, cost-utility analysis, cost-effectiveness analysis, cost-consequence analysis, and cost-minimization analysis Language Studies published in Spanish or English Publication type Only full original publications GAE: genicular artery embolization; HRQoL: health related quality of life; ICER: incremental cost-effectiveness ratio; KO: knee osteoarthritis; LYG: life years gained; QALY: quality-adjusted life years.
- 3)
Study selection, data extraction process and assessment of risk of bias
Two reviewers independently and in parallel performed the selection process, data extraction, and assessment of risk of bias. Titles and abstracts were initially assessed, followed by a full-text review for studies meeting the criteria. A standardized data extraction form was created in Microsoft Excel, including information on article identification, study design, participant characteristics, eligibility criteria, intervention and comparator details; assessed effectiveness and safety outcomes along with measurement timing; cost-effectiveness methodology and outcomes; study funding source, and disclosed conflicts of interest by the researchers.
To assess the methodological quality of the identified systematic reviews, the AMSTAR-2 tool was selected.24 The risk of bias of the included primary studies was assessed using the Cochrane RoB 2 tool for parallel and crossover randomized controlled trials.25 The appraisal of methodological quality of economic evaluations was planned based on Drummond et al.’s criteria list.26
Discrepancies between reviewers were resolved through discussion.
- 4)
Publication bias assessment
The assessment of publication bias was planned by creating a funnel plot and computing the Egger's regression test;27 however, the minimum number of studies necessary was not reached.
- 5)
Synthesis of the evidence
Study characteristics were summarized narratively and presented in summary tables. Meta-analyses were conducted for quantitative synthesis. Heterogeneity was assessed using forest plots, the χ2 statistical (p<0.01) and the Higgins I2 test. Initially, a fixed-effect model was used to evaluate the statistical heterogeneity among included studies (I2 statistics). In cases of high unexplained heterogeneity (I2>70%), meta-analyses was not performed, and results were reported narratively. All analyses were conducted using RevMan 5.4.28
Potential confounders considered were baseline pain level, osteoarthritis severity, and type of embolic agents used. However, subgroup analyses were limited due to the small number of studies evaluated.
- 6)
Certainty of evidence
The certainty of the evidence for all outcomes was judged using the GRADE methodology, considering risk of bias, consistency, directness, precision and reporting bias.29 Certainty was assessed as high, moderate, low or very low. A summary of findings table was prepared to present the certainty of the evidence and the magnitude of the effects for the main comparison.30,31
A cost-analysis was conducted to compare the costs (in Euros of 2023) of two treatments for chronic pain secondary to KO: the use of GAE of the affected knee followed by standard treatment (evaluated strategy), and the standard treatment alone (comparator), which involves annual hyaluronic acid injections and prescription of physical exercise, in addition to radiofrequency sessions if the patient does not improve.
The target patients were those with chronic pain secondary to mild to moderate KO refractory to standard treatment or non-surgical candidates with severe KO. The analysis was performed from the perspective of the Spanish National Health System (NHS) with a one-year time horizon covering the entire treatment period in the evaluated strategy. No discount rate was applied due to the short-term horizon.
The incremental cost per patient and the annual cost for a hospital with a target population size of 40 patients (value reported by experts) were calculated. Additionally, we estimated the health improvement, in quality-adjusted life years (QALY), that GAE should generate per patient to be considered a cost-effective technology. This was estimated by solving the incremental effectiveness EA−EB from the incremental cost-effectiveness ratio equation (ICER=CA−CB/EA−EB, where CA−CB is the estimated incremental cost, and A and B represent the evaluated strategy and the comparator, respectively). The cost-effectiveness threshold estimated for Spain (€ 25 000 per QALY)32 was applied. The analysis was conducted using Microsoft Excel 2013.
We performed one-way deterministic and probabilistic (1000 second-order Monte-Carlo simulations) sensitivity analyses.
- 1)
Parameters
Since both alternatives include the standard treatment, only the resource use and the corresponding costs of the GAE were considered, as the inclusion of shared costs would not affect the incremental cost.26
The analysis included those associated with the intervention (drugs, instruments, wound closure, healthcare personnel, operating room use, and post-intervention observation period) and the follow-up (Appendix B in Supplementary data).
ResultsSystematic review on effectiveness and safetyThe search in Epistemonikos yielded a total of 13 systematic reviews. Nine were initially considered potentially eligible after title and abstract screening according to the selection criteria; however, none were finally included because they did not attain a high-quality rating.4,6,13–16,33–35 The full quality assessment can be found in Appendix C in Supplementary data.
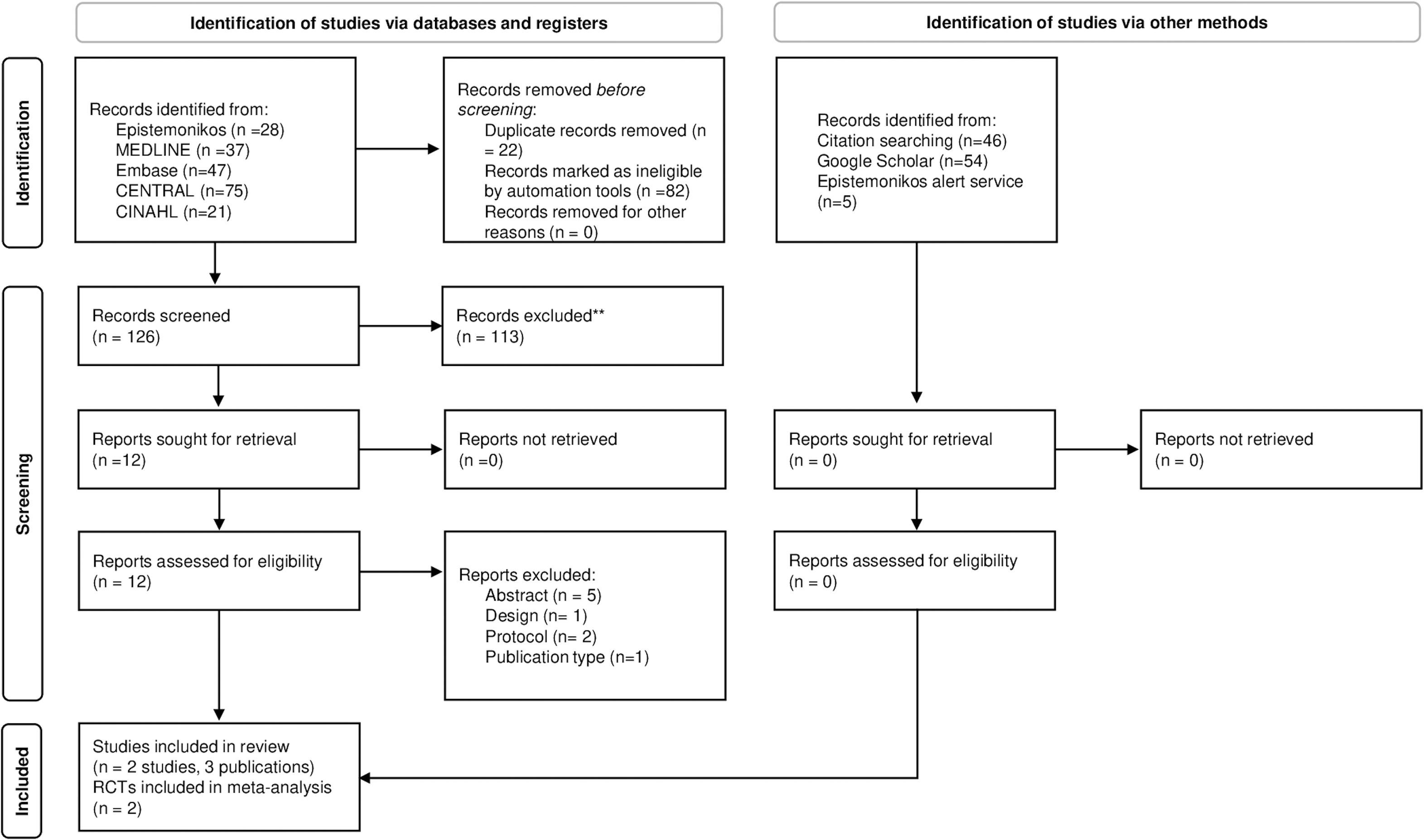
The search for primary studies retrieved 126 references after removing duplicates (Fig. 1). After the title and abstract screening, 12 publications were selected for full-text analysis. According to pre-established criteria, nine of these were excluded. Appendix D in Supplementary data shows the list of excluded references and the main reason for exclusion.
Examination of the bibliographic listing of included studies and the Google Scholar search did not lead to any additional studies. No additional studies were identified through the alert service created using the Epistemonikos database. Therefore, the final selection consisted of two randomized controlled trials,36,37 reported in three publications.36–38
- 1)
Characteristics of included studies
The main characteristics of the included studies are summarized in Tables 2 and 3. For a detailed description of the characteristics and further details of the included studies, please refer to Appendix E and F in Supplementary data.
Table 2.Selection criteria and baseline characteristics of participants in the included studies.
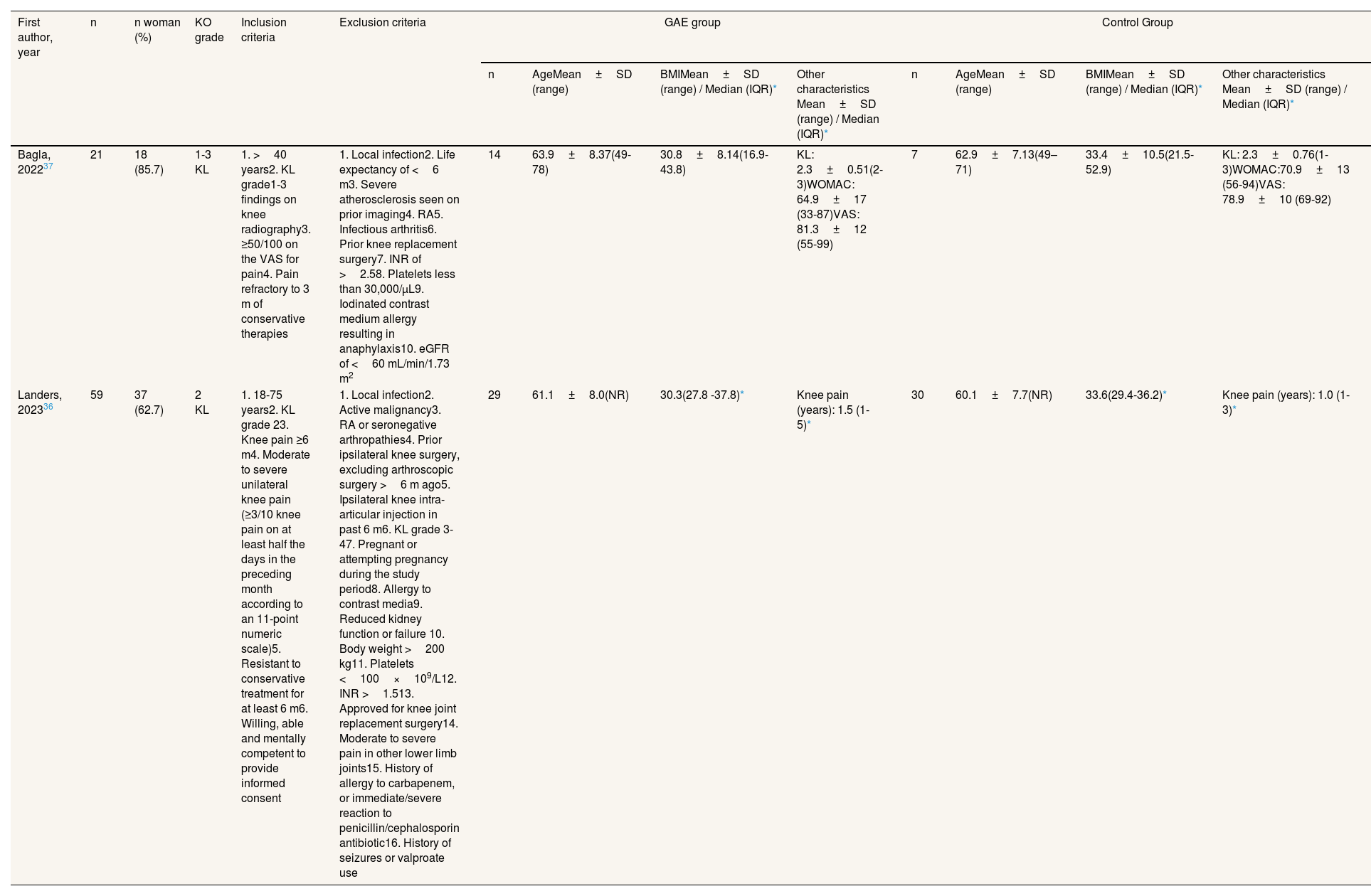
First author, year n n woman (%) KO grade Inclusion criteria Exclusion criteria GAE group Control Group n AgeMean±SD (range) BMIMean±SD (range) / Median (IQR)* Other characteristics Mean±SD (range) / Median (IQR)* n AgeMean±SD (range) BMIMean±SD (range) / Median (IQR)* Other characteristics Mean±SD (range) / Median (IQR)* Bagla, 202237 21 18 (85.7) 1-3 KL 1. >40 years2. KL grade1-3 findings on knee radiography3. ≥50/100 on the VAS for pain4. Pain refractory to 3 m of conservative therapies 1. Local infection2. Life expectancy of <6 m3. Severe atherosclerosis seen on prior imaging4. RA5. Infectious arthritis6. Prior knee replacement surgery7. INR of >2.58. Platelets less than 30,000/μL9. Iodinated contrast medium allergy resulting in anaphylaxis10. eGFR of <60 mL/min/1.73 m2 14 63.9±8.37(49-78) 30.8±8.14(16.9-43.8) KL: 2.3±0.51(2-3)WOMAC: 64.9±17 (33-87)VAS: 81.3±12 (55-99) 7 62.9±7.13(49–71) 33.4±10.5(21.5-52.9) KL: 2.3±0.76(1-3)WOMAC:70.9±13 (56-94)VAS: 78.9±10 (69-92) Landers, 202336 59 37 (62.7) 2 KL 1. 18-75 years2. KL grade 23. Knee pain ≥6 m4. Moderate to severe unilateral knee pain (≥3/10 knee pain on at least half the days in the preceding month according to an 11-point numeric scale)5. Resistant to conservative treatment for at least 6 m6. Willing, able and mentally competent to provide informed consent 1. Local infection2. Active malignancy3. RA or seronegative arthropathies4. Prior ipsilateral knee surgery, excluding arthroscopic surgery >6 m ago5. Ipsilateral knee intra-articular injection in past 6 m6. KL grade 3-47. Pregnant or attempting pregnancy during the study period8. Allergy to contrast media9. Reduced kidney function or failure 10. Body weight >200 kg11. Platelets <100×109/L12. INR >1.513. Approved for knee joint replacement surgery14. Moderate to severe pain in other lower limb joints15. History of allergy to carbapenem, or immediate/severe reaction to penicillin/cephalosporin antibiotic16. History of seizures or valproate use 29 61.1±8.0(NR) 30.3(27.8 -37.8)* Knee pain (years): 1.5 (1-5)* 30 60.1±7.7(NR) 33.6(29.4-36.2)* Knee pain (years): 1.0 (1-3)* BMI: body mass index; eGFR: estimated glomerular filtration rate; GAE: genicular artery embolization; IQR: interquartile range; INR: International Normalized Ratio; KO: knee osteoarthritis; KL: Kellgren-Lawrence; NR: not reported; RA: rheumatoid arthritis; SD: standard deviation; VAS: visual analogue scale; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index.
Table 3.Characteristics of genicular artery embolization and control procedures in the included studies.
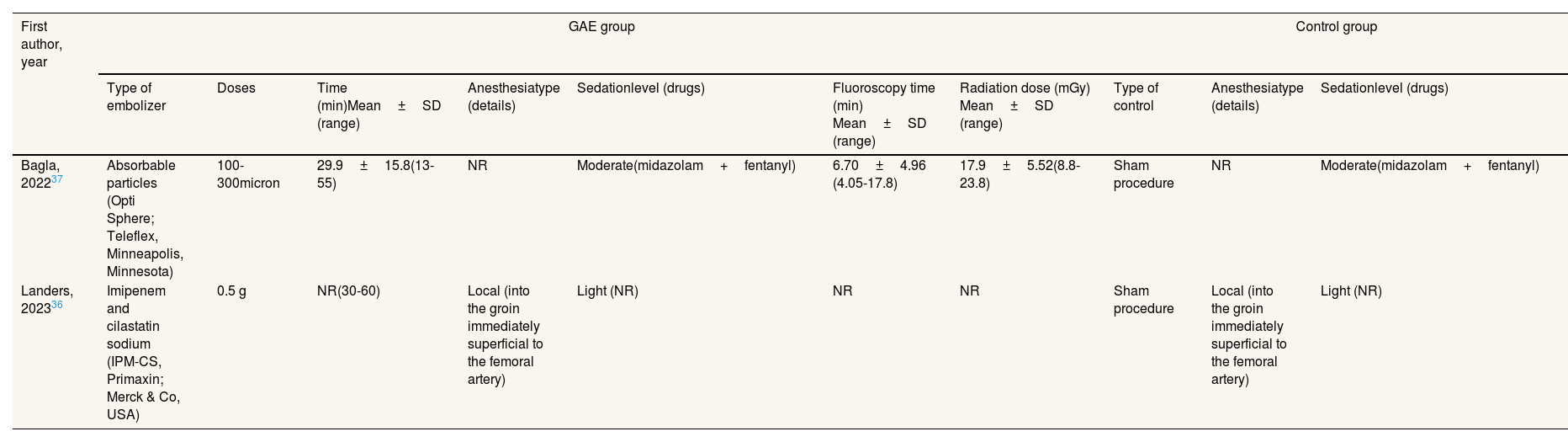
First author, year GAE group Control group Type of embolizer Doses Time (min)Mean±SD (range) Anesthesiatype (details) Sedationlevel (drugs) Fluoroscopy time (min) Mean±SD (range) Radiation dose (mGy) Mean±SD (range) Type of control Anesthesiatype (details) Sedationlevel (drugs) Bagla, 202237 Absorbable particles (Opti Sphere; Teleflex, Minneapolis, Minnesota) 100-300micron 29.9±15.8(13-55) NR Moderate(midazolam+fentanyl) 6.70±4.96 (4.05-17.8) 17.9±5.52(8.8-23.8) Sham procedure NR Moderate(midazolam+fentanyl) Landers, 202336 Imipenem and cilastatin sodium (IPM-CS, Primaxin; Merck & Co, USA) 0.5 g NR(30-60) Local (into the groin immediately superficial to the femoral artery) Light (NR) NR NR Sham procedure Local (into the groin immediately superficial to the femoral artery) Light (NR) GAE: genicular artery embolization; NR: not reported; SD: standard deviation.
- 2)
Risk of bias in included studies
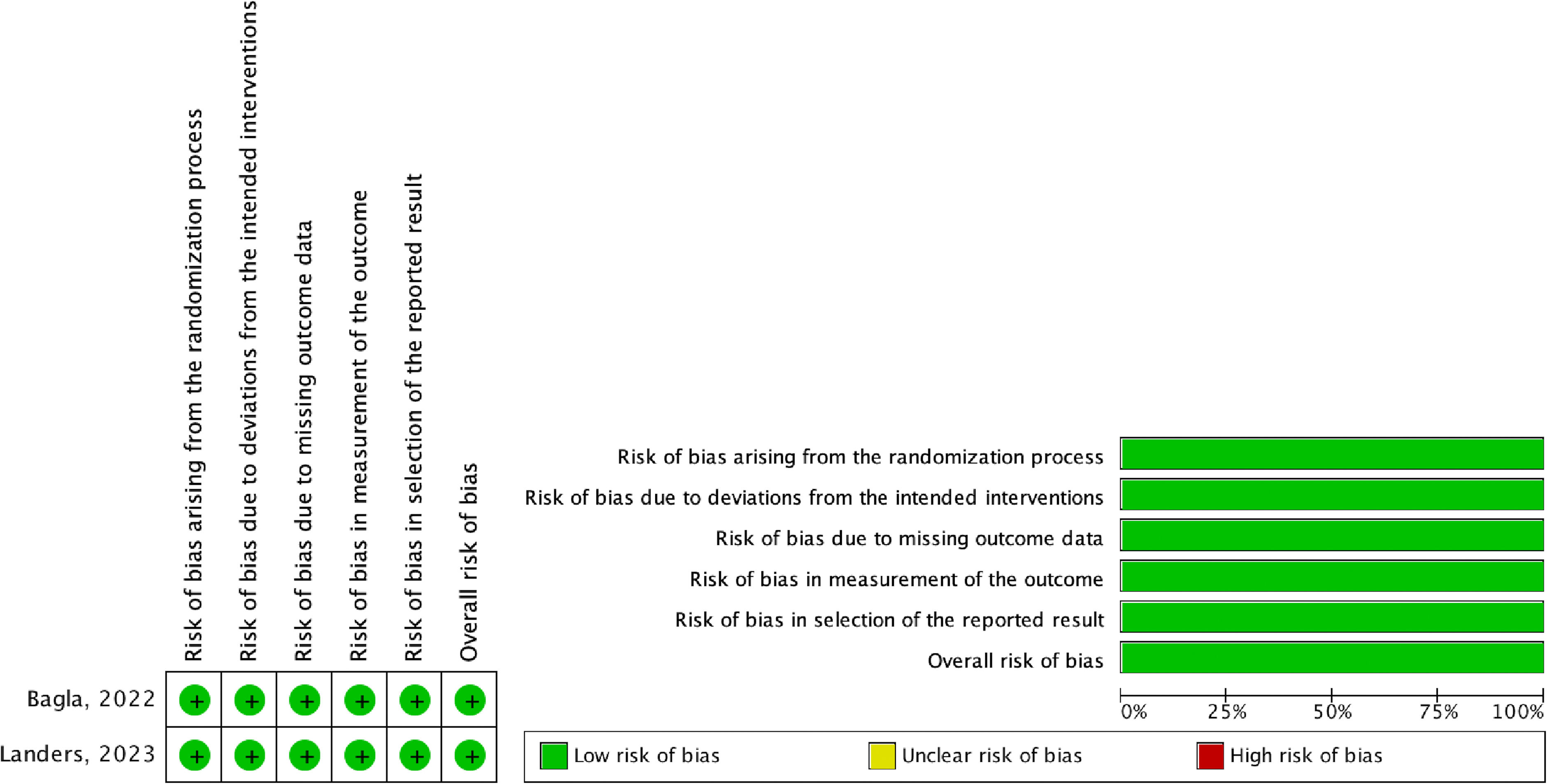
The overall risk of bias in the included randomized controlled trials was considered low. The summary of the assessment can be found in Figure 2 and Appendix G in Supplementary data.
- 3)
Certainty of evidence
The overall quality of evidence was considered low. The evidence profile for GAE vs. standard treatment outcomes indicated moderate to low certainty of the evidence (Appendix H in Supplementary data).
- 4)
Summary of results
Results of all meta-analysis conducted are available in Appendix I in Supplementary data.
- •
Pain (certainty of the evidence: low ⊕⊕⊖⊖/moderate ⊕⊕⊕⊖)
The included randomized controlled trials examined the effect of GAE on pain at 1 month,36,37 6 months36, and 12 months.36 However, due to very high heterogeneity rates (I2=91%) in the 1-month pain analysis, pooled data are not presented.
Preliminary findings reveal significant discrepancies in pain levels 1 month after GAE. There is low-quality evidence suggesting that GAE may have little to no effect according to the KOOS Pain subscale (1 study; 59 patients)36 or may result in a slight decrease in pain levels assessed with the visual analogue scale (VAS) (mean difference [MD]=50.1mm; 95% confidence interval [95%CI]: 29.0-72.3; 1 study; 21 patients).37 At 6 and 12 months of follow-up, moderate quality evidence suggests GAE probably results in little to no effect.
In the Bagla et al.37 study, the response rates at 1 month were 79% (11/14) and 0% (0/7) for the GAE and sham arms, respectively. However, in the Landers et al.36 study, overall change in knee pain at 12 months indicated that 17 participants (58.6%) in the GAE group reported being moderately or much better, compared to 11 participants (37.9%) in the control group, though this difference was also not statistically significant.
- •
Overall function (certainty of the evidence: moderate ⊕⊕⊕⊖)
The included studies examined the impact of interventions (GAE vs. sham) on overall functional improvement at 1 month,36,37 6 months,36 and 12 months,36 using the WOMAC,37 KOOS Daily Living subscale, and KOOS Function in Sport and Recreation subscale.36 The effects in all these scales were pooled.
Moderate-quality evidence suggests GAE likely has little to no effect on functional capacity at 1 month (SMD=−0.18; 95%CI: −0.62-0.27; I2=0%, 2 studies, 80 patients), 6 months (SMD=−0.17; 95%CI: −0.68-0.34, 1 study, 59 patients) and 12 months (SMD=0.07; 95%CI: −0.44- 0.58, 1 study, 59 patients) compared to standard or pharmacological treatment.
- •
Health related quality of life (HRQoL) (certainty of the evidence: low ⊕⊕⊖⊖/moderate ⊕⊕⊕⊖)
Only Landers et al.36 reported on the effect of interventions on HRQoL. Low-quality evidence suggests that GAE may have little to no effect on HRQoL assessed with the KOOS Quality of Life subscale at 1 month compared to standard treatment (1 study, 59 patients). GAE probably results in little to no difference in HRQoL at 6 and 12 months.
However, low-quality evidence suggests that GAE may result in a slight improvement of HRQoL levels using the EQ-5D VAS at 6-month follow-up (MD=−10.00; 95%CI: −19.45 to −0.55; 1 study, 59 patients). Moderate-quality evidence suggests that GAE likely has little to no effect on HRQoL at 1 month and 12 months compared to standard treatment (1 study, 59 patients).
No differences were observed in the rates of patients who did not present or presented slight problems with anxiety, discomfort, mobility, usual activities, or self-care assessed with EQ-5D.
- •
Need for pain medication (certainty of the evidence: moderate ⊕⊕⊕⊖)
Only Landers et al.36 reported changes in the need for pain medication. At 12 months, the GAE group had a lower proportion of participants taking analgesics (control 48%, intervention 24%). However, moderate-quality evidence suggests that GAE likely does not reduce or increase the need for pain medication (risk ratio: 0.52; 95%CI: 0.24-1.10; 1 study, 59 patients).
- •
Adverse events and complications
No major adverse events were reported in either of the two studies.36,37 Specifically, in the study by Landers et al.,36 it was reported that no evidence of osteonecrosis or ischemic complications were found on magnetic resonance imaging up to two years following the procedure. No differences were observed in minor adverse events.36,37
The electronic databases retrieved nine references, but after reading titles and abstracts, all of them were excluded (Appendix J in Supplementary data). Therefore, no economic evaluations focused on GAE and that meet the established inclusion criteria were identified.
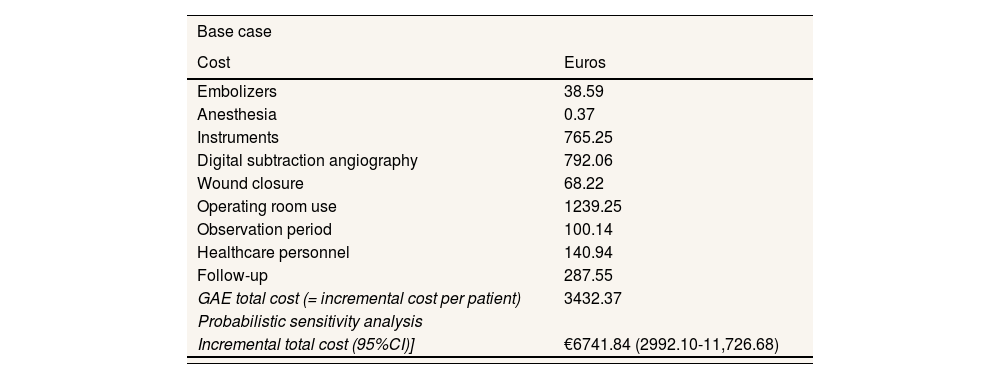
Economic evaluationOver a one-year horizon, the inclusion of GAE in the standard treatment generates an increment in costs of € 3432.37 per patient, from the NHS perspective (Table 4). Considering this incremental cost, a health improvement of 0.137 QALY per patient is required for GAE to be deemed cost-effective compared to the standard treatment. In addition, the estimated annual cost of implementing this technology would be € 137 294.86 for a general hospital treating a target population of 40 patients per year.
Results of the cost analysis: base case and sensitivity analysis. Costs per patient.
| Base case | |
|---|---|
| Cost | Euros |
| Embolizers | 38.59 |
| Anesthesia | 0.37 |
| Instruments | 765.25 |
| Digital subtraction angiography | 792.06 |
| Wound closure | 68.22 |
| Operating room use | 1239.25 |
| Observation period | 100.14 |
| Healthcare personnel | 140.94 |
| Follow-up | 287.55 |
| GAE total cost (= incremental cost per patient) | 3432.37 |
| Probabilistic sensitivity analysis | |
| Incremental total cost (95%CI)] | €6741.84 (2992.10-11,726.68) |
| One-way deterministic sensitivity analysis | |||
|---|---|---|---|
| Parameter | Value in base case | New value | Cost of GAE(= incremental cost per patient) |
| Patients with allergy to antibiotics (%) | 15 | 12 [Asm, −20%] | 3426.67 |
| 18 [Asm, +20%] | 3438.07 | ||
| Patients needed a vascular closure of the wound (%) | 1 | 5 [Asm] | 3433.86 |
| 10 [Asm] | 3435.71 | ||
| GAE sessions (per year) | 1 | 2 [Experts] | 6577.20 |
| 3 [Experts] | 9722.02 | ||
| GAE duration (hours) | 1.5 | 1 [Experts] | 2884.14 |
| 2 [Experts] | 3866.60 | ||
| Observation period (hours) | 4 | 3.2 [Asm, −20%] | 3412.34 |
| 4.8 [Asm, +20%] | 3452.40 | ||
| Follow-up visits | 3 | 2 [Asm] | 3336.52 |
| 4 [Asm] | 3528.22 | ||
| Cost of usual embolizer (€ per vial) | 10.10 | 8.08 [Asm, −20%] | 3430.65 |
| 12.12 [Asm, +20%] | 3434.09 | ||
| Cost of embolizer used in case of antibiotic allergy (€ per vial) | 200 | 160 [Asm, −20%] | 3426.37 |
| 240 [Asm, +20%] | 3438.37 | ||
| Cost of anesthesia (€ per ampoule) | 0.37 | 0.22 [Min45] | 3432.22 |
| 0.67 [Min45] | 3432.67 | ||
| Cost of wound closure by manual compression | 67.85 | 54.28 [Asm, −20%] | 3418.94 |
| 81.42 [Asm, +20%] | 3445.81 | ||
| Cost of vascular closure of the wound | 105 | 84 [Asm, −20%] | 3432.16 |
| 126 [Asm, +20%] | 3432.58 | ||
| Cost of digital subtraction angiography | 792.06 | 428.85 [Min46] | 3069.16 |
| 1054 [Max46] | 3694.31 | ||
| Cost of 5 Fr introducer | 97.01 | 13.33 [Min42–44] | 3348.69 |
| 193.88 [Max42–44] | 3529.24 | ||
| Cost of 5 Fr catheter (cobra) | 38.17 | 19.27 [Min42,44] | 3413.47 |
| 111.63 [Max42,44] | 3505.83 | ||
| Cost of microcatheter | 368.82 | 311.38 [Min42,44] | 3374.92 |
| 456.40 [Max42,44] | 3519.95 | ||
| Cost of 0.35 microguide | 261.25 | 209 [Asm, −20%] | 3380.12 |
| 313.51 [Asm, +20%] | 3484.62 | ||
| Cost of the observation period (€ per hour) | 25.04 | 4.67 [Min46] | 3350.90 |
| 51.46 [Max46] | 3538.06 | ||
| Labor cost (€ per hour) | 31.32 | 25.06 [Asm, −20%] | 3404.18 |
| 37.58 [Asm, +20%] | 3460.56 | ||
| Cost of follow-up visit | 95.85 | 40.02 [Min46] | 3264.89 |
| 153.04 [Max46] | 3603.95 | ||
| Cost of operating room (€ per session of 1.5 hours) | 1239.25 | 991.4 [Asm, −20%] | 3184.52 |
| 1487.1 [Asm, +20%] | 3680.22 | ||
Asm: assumption; 95%CI: 95% confidence interval; GAE: genicular artery embolization.
The one-way sensitivity analysis shows that the incremental cost can vary from € 2884.14, if the intervention duration is reduced from 1.5hours to 1 hour, to € 9722.02, if GAE is administered three times to the same patient within the time horizon. Meanwhile, the probabilistic analysis estimates an incremental cost of € 6741.84 per patient (95%CI: 2992.10-11,726.68), associated with the application of the GAE technique (Table 4).
DiscussionThe present systematic review on the effectiveness and safety of GAE for chronic pain secondary to KO refractory to standard treatment identified two randomized controlled trials (n=80) evaluating GAE compared to standard treatment.
The results show that evidence is insufficient to draw definitive conclusions regarding the beneficial effects of GAE compared to standard treatment for KO in terms of knee pain, overall functional improvement, HRQoL, changes in the need for pain medication, adverse events or complications. Evidence for these outcomes is affected by inconsistency and imprecision, with wide confidence intervals and/or a very small sample size.
Recent research has shown promising results for GAE as an alternative treatment for chronic joint pain in patients with KO.4,13–16 Some studies suggest that GAE can offer benefits across varying degrees of osteoarthritis severity,13 while others consider that this technique has potential mainly for early and low-grade osteoarthritis, particularly in the short and medium term.14 Additionally, this procedure is considered safe, with no serious complications reported,4,6,13 but minor complications are not uncommon.13
However, these findings are predominantly based on observational studies, highlighting the need to confirm them with high-quality randomized controlled trials. Further research is needed to evaluate GAE long-term outcomes, its comparative efficacy with other modalities, and its role in the therapeutic approach.4,6,16,35
The present systematic review findings suggest that GAE appears to be a safe alternative, as previous studies have indicated, but without evidence of its effectiveness within a one-year timeframe. The above-mentioned limitations, and considering the low certainty evidence supporting our conclusions, justify keeping this question in “living mode” as proposed in our original protocol.39
The systematic review did not identify any economic evaluation on GAE that met the established inclusion criteria. The identified evidence is insufficient to establish significant differences between the GAE technique and the comparator (necessary to perform a cost-effectiveness analysis) or to conclude that both treatments have the same effectiveness (necessary to perform a cost-minimization analysis). Therefore, we only compared the costs conducting a cost-analysis.
The cost-analysis results showed that the incorporation of GAE into the standard treatment (application of hyaluronic acid once a year plus a prescribed physical exercise regimen) would lead to an increase in cost of € 3432.37 per patient compared to the standard treatment alone in a year. This translates to an additional annual cost of € 137,294.86 for a general hospital with a target population of 40 patients per year. This technology could only be considered cost-effective if an improvement in health of at least 0.137 QALY per patient within a year is achieved.
This systematic review has several strengths. It was developed using a robust predefined methodology outlined in a registered protocol. All steps were performed in duplicate to minimize errors throughout the review process. Additionally, the evidence was synthesized using the GRADE methodology, known for its transparency in evidence development and presentation.
However, the main limitation of this review is the scarcity of evidence, stemming from a low number of studies with small sample sizes, leading to inconsistent results in measured outcomes. Others, mainly due to the low number of studies, include the absence of sensitivity, subgroup and meta-regression analyses. Additionally, as is standard in systematic reviews, the methodology may have excluded unpublished studies, published in languages other than English or Spanish, or in unindexed journals, which could potentially limit the comprehensiveness of the evidence. Furthermore, although an alert service for identifying randomized controlled trials and non-randomized comparative studies remained active until May 31, 2024, a gap exists between this date and the present, which may have excluded more recent evidence potentially influencing the findings.
To the best of the authors’ knowledge, this is the first analysis assessing the costs of the GAE technique in Spain. A complete economic evaluation comparing costs and effects could not be performed due to effectiveness results. However, given the usefulness of ICER, as an efficiency measure, in the decision-making process, we estimated the health improvement per patient necessary for GAE to be considered cost-effective, compared to the standard treatment, for a willing-to-pay threshold of € 25,000 per QALY.
The main limitations of the cost analysis arise from data scarcity. First, evidence on the proportion of target patients requiring vascular closure or those allergic to antibiotics could not be identified, thus assumptions and a value for a general patient40 were used, respectively. Second, the cost of wound closure with manual compression was obtained from a single study without specifying the year in which the Euros were measured.41 Therefore, 2020 (the publication year) was assumed as the reference year. Third, instrument costs were extracted from tender documents of some Spanish public hospitals,42–44 reflecting their reality but may vary contextually. Fourth, resource use information was provided by an expert. Although clinical practice was assumed to be at least similar across Spain, there could be regional differences. Sensitivity analyses show that the incremental cost per patient can range from € 2884.14 to € 11 726.68, a wide variation especially influenced by the number of sessions conducted during the time horizon. Bearing in mind this, regional differences in terms of incremental costs related to the use of resources derived from the clinical practice and the unit costs in each region could be observed. Nonetheless, experts validated the assumptions (face validity) and sensitivity analyses variations of these parameters did not significantly alter the results. Finally, the time horizon was restricted to one year because there is no evidence on effectiveness and safety beyond this period, so the impact on resource use is unknown. In addition, a previous study suggests that the benefits of GAE are particularly noticeable in the short and medium term.14
ConclusionThe present study suggests that there is no difference between GAE and control groups in terms of effectiveness and safety, but it is more costly than the standard treatment in Spain. Larger randomized controlled trials are necessary to elucidate the effects of GAE for chronic pain secondary to KO and, consequently, to determine whether it could potentially become cost-effective.
Genicular artery embolization has emerged as a promising minimally invasive procedure for refractory knee osteoarthritis, but evidence on its safety, effectiveness and cost-effectiveness compared to standard care is still uncertainty.
What does the study add to the literature?Genicular artery embolization appears safe, but its effectiveness over standard treatment for knee osteoarthritis is unclear, and its use generates and additional € 3432.37 per patient, requiring 0.137 quality-adjusted life years improvement to be cost-effective.
What are the implications of the results?Genicular artery embolization is more costly than standard care in Spain and requires specific health improvements to be cost-effective; larger studies are needed to confirm its viability as a cost-effective treatment for knee osteoarthritis.
A. Hernández-Yumar and Y. González-Hernández participated in the design, acquisition, analysis, and interpretation of data, as well as drafting the work. These authors share first authorship. T. del Pino-Sedeño, C. Valcárce-Nazco, A. de Armas-Castellano and E. Herrera-Ramos participated in the design, acquisition, analysis, and interpretation of data, and reviewed the work. J. Portero Navarro, M. Carmona Rodríguez, M.X. Rojas-Reyes and M.M. Trujillo-Martín participated in the design, and critically reviewed the work. T. del Pino-Sedeño and M.M. Trujillo-Martín also contributed to project administration. All authors read and approved the final manuscript.
FundingThis study was commissioned and funded by the Spanish Ministry of Health in the framework of activities carried out by the Spanish Network of Agencies for Health Technology Assessment and Services for the National Health System (RedETS). Additionally, this work received support from the Living Evidence to Inform Health Decisions (LE-IHD) program's project entitled “Strengthening decision-making capacity in the Spanish Health System through living evidence: An innovative framework” that has been funded by Instituto de Salud Carlos III (ISC-III) Grant PI21/01564. The study was conducted independently of study sponsors. There was no sponsor involvement in the study design; collection, analysis and interpretation of the data; in writing of the manuscript; or in the decision to submit the manuscript for publication.
Conflicts of interestNone.