To compare the use of health technology assessment (HTA) as a tool to support pricing and reimbursement (P&R) of new medicines in Spain with England, Sweden, France and Germany.
MethodFor each country, the literature is used to identify the purpose and timing of the P&R decision, the HTA and decision-making procedures used to generate evidence, and the criteria used to make decisions.
ResultsResults are presented as a summary of the HTA landscape for P&R of new medicines in each country. Comparisons are made between Spain and other countries regarding the procedure and implementation of HTA.
ConclusionsBased on these assessments, we made recommendations for how HTA might develop in Spain with the aim of improving governance and efficiency. Spain has made considerable progress in recent years, but still falls short of international standards in terms of independence of the HTA agencies and decision-making committees from political influence and industrial policy, the setting of prices of medicines in relation to health gain, improve the transparency of the process and results of the evaluation, and promote the participation of stakeholders. In common with other countries, Spain needs to clarify the role of cost-effectiveness criteria. Further progress needs to be made to coordinate effort across the various agencies, strengthen technical staff, and ensure equitable access to medicines between regions.
Comparar el uso de la evaluación de tecnologías sanitarias (ETS) como instrumento para apoyar la fijación de precios y el reembolso de nuevos medicamentos en España con otros países europeos como Inglaterra, Suecia, Francia y Alemania.
MétodoSe realiza una revisión de la literatura para identificar, en cada país, el objetivo y la cronología de la decisión, los procedimientos para realizar la ETS y los criterios para tomar decisiones.
ResultadosSe presenta una descripción narrativa de la situación en cada país y una comparación entre España y otros países sobre los procedimientos y la implementación de ETS.
ConclusionesBasándose en estas evaluaciones, se propone una serie de recomendaciones para mejorar el proceso. España ha avanzado significativamente en ETS en los últimos años, pero aún falta garantizar la independencia de las agencias de ETS, eliminar la influencia de la política económica e industrial de los comités de decisiones sanitarias, fijar precios basados en el valor terapéutico, mejorar la transparencia del proceso y de los resultados de las evaluaciones, y por último, aumentar la participación de los grupos de interés. Al igual que en otros países de su entorno, España tiene que clarificar el papel de la evaluación económica como criterio de decisión. Hace falta una mejor coordinación entre las diversas agencias que participan en ETS en España, fomentar el personal técnico y monitorizar la equidad de acceso a nuevos medicamentos entre las comunidades autónomas.
The market for medicines in Europe is highly regulated, diverse and complex. The European Commission establishes common procedures for marketing authorization, and broad principles for pricing and reimbursement (P&R) (such as the need for criteria to be clear and objective),1 but national authorities set the criteria they consider appropriate.
This article compares P&R in Spain with four other countries: England, Sweden, France, and Germany. The process in Spain has recently been criticized by public auditors,2,3 particularly concerning the vagueness of the criteria for P&R decisions and the high degree of discretion allowed to decision making bodies and there have been several proposals for reform4–7 A substantial literature has compared health technology assessment (HTA) procedures across countries8–16 but to our knowledge this is the first study that makes recommendations specifically for the P&R process for new medicines for Spain. The four comparator countries were chosen because they each have mature HTA systems (and hence have had time to learn from experience), offer universal public healthcare (as does Spain), and have formal, well-respected HTA processes. Nevertheless, HTA takes place within a particular institutional, legal and cultural setting, and hence comparisons must be cautious.
MethodWe searched the official documentation for HTA procedures for supporting P&R available from public websites of the relevant country agencies during July 2018. This was supplemented by country profiles from the World Health Organization or the Organization for Economic Collaboration and Development. We cross-checked and filled in gaps from two recent reviews.8,10 For the comparator countries we included only documents in English. This was not an important handicap as these countries include extensive English documentation on their websites. For Spain, we included documents in English or Spanish. We examine how the health service is financed and organized, the purpose and timing of the P&R decision, the HTA procedures supporting that decision, and the criteria used. Results are presented as summary tables and narrative synthesis.
ResultsTable 1 gives a short description of the financing and organization of healthcare services in each country.17–21Table 2 summarises the timing and purpose of HTA in each country, and Table 3 describes the criteria used to make P&R decisions. Spain and France use HTA to support decisions for P&R before any medicine can be sold in the country. Sweden only uses HTA to support P&R for outpatient medicines. England allows free-pricing (with profit control), and conducts HTA to support reimbursement decisions, while Germany operates free-pricing with full reimbursement for up to 12 months, with HTA supporting subsequent P&R decisions.
Financing and organization of health services.
| Means of funding | Responsibility for health service provision | Organization of health service | Access to specialists and choice of provider | Ownership of hospitals which provide services in the public health system | |
|---|---|---|---|---|---|
| England19 | General taxationa | Local agencies. No local tax-raising powers | Centralized. Some hospitals granted autonomy | Gate-keeper GPs. Limited choice of provider | Mainly public ownership. Some PPC |
| Spain20 | General taxationa | Devolved to 17 regions. Regions have limited tax-raising powers | Centralized within each region | Gate-keeper GPs. Limited choice of provider | Mainly public ownership. Some PPC |
| Sweden21 | Local taxationb | Local councils decide taxation and public spending in their region | Varies by county | Degree of choice varies across counties. | Mainly public ownership |
| France22 | Social insuranceb | Not-for-profit health insurers | Loosely organised | Direct access to specialists (with a fee). Choice of provider | Public and private ownership |
| Germany23 | Social insuranceb | Not-for-profit health insurers | Loosely organised | Direct access to specialists (with a fee). Choice of provider | Public and private ownership |
GPs: general practitioners; PPC: public-private collaboration.
Summary of purpose and timing of health technology appraisal in each country.
| Price setting (if price is regulated) and reimbursement decisionsa | Implementation of decision in clinical practice and revision | |
|---|---|---|
| England | No regulated price of drug. NICE conducts HTA to decide on reimbursement of selected drugs and devices | NICE decisions legally binding on the NHS. Decision often reviewed after about 5 years |
| Sweden | TLV conducts HTA for price and reimbursement of all new outpatient and high cost medicines. Local councils decide on reimbursement for inpatient drugs | TLV decision legally binding on local councils. Decision may be reviewed after 5 years |
| Spain | AEMPS conducts clinical HTA on some new drugs (IPT). MoH (Pharmacy General Director) negotiates price. CIPM makes final decision on P&Rb | The 17 autonomous regions and hospital pharmacies may conduct further HTAc |
| France | TC conducts clinical HTA on all new drugs. MoH decides price. Health insurers (UNCAM) decide reimbursement | TC revises the decision every 5 years for reimbursed drugs |
| Germany | Free pricing for the first 12 months.IQWiG conducts clinical HTA on all new drugs. GB-A decides level of added benefit. Health insurers (GKV-SV) negotiate price and decided reimbursement | Revision of the decision is made at least one year after benefit assessment |
AEMPS: Spanish Agency for Evaluation of Medicines and Healthcare Products; CIPM: Interministerial Committee for Pricing; G-BA: Federal Joint Committee; GKC-SV: National Association of Statutory Health Insurance Funds; HAS: Haute Autorité de Santé; HTA: health technology appraisal; IPT: Therapeutic Position Report; IQWiG: Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen; MoH: Ministry of Health or equivalent government department; NHS: National Health Service; NICE: National Institute for Health and Care Excellence; TC: Transparency Commission, Economic Evaluation and Public Health Commission; TLV: Tandvårds- och läkemedelsförmånsverket; UNCAM: National Association of Health Insurance Funds.
Summary of criteria for P&R decisions based on health technology appraisal assessments.
| Criteria used for decision making | |
|---|---|
| England | Incremental cost per QALY. An explicit threshold is applied. Other factors may also be taken into account |
| Sweden | Incremental cost-per-QALY. No explicit (but implicit) threshold. The criteria of “human value” and “need and solidarity” also have to be met (societal perspective) |
| Spain | CIPM decides price and reimbursement “taking into account” mainly therapeutic usefulness, budget impact, and cost-effectiveness |
| France | Inpatient drugs included in the positive list are reimbursed at 100%. For outpatient drugs, the reimbursement rate (between 15% and 65%) depends on whether the actual benefit level is judged mild, moderate or important. The price depends on the improvement in actual benefit offered by the drug (ASMR scale I – V). The manufacturer submits a cost-effectiveness analysis for innovative drugs (ASMR I to III). No threshold cost-per-QALY |
| Germany | Price and reimbursement depends on whether the added benefit level is judged major, considerable or mild, and the strength of evidence. IQWIG are still considering whether or how economic evaluation should be applied in HTA in Germany |
ASMR: level of improvement of clinical benefit; CIPM: Interministerial Committee for Pricing; HTA: health technology appraisal; IQWIG: Institut für Qualität und Wirtschaftlichkeit im Gesundheitswesen; QALY: quality-adjusted life-year.
Responsibility for provision of healthcare is for the most part decentralized to the 17 regions. However, before any new medicine can be marketed, the decisions for P&R are made centrally by the Interministerial Committee on Pricing of Medicines and Healthcare Products (CIPM). This body includes nominees from the Ministries of Health, Industry, Economics and Finance, plus members from three of the regions on a rotating basis. Legally, regions are not supposed to deny access to medicines with centrally approved reimbursement but in practice, within their role of managing and paying healthcare providers, some regional payers apply further criteria.
Spain operates two separate sets of HTA agencies, each with distinct roles. The Spanish Agency for Medicines and Healthcare Products (AEMPS) conducts clinical HTA for new medicines. The Regional Network (AETS) evaluates the effectiveness and costs of other health technologies, e.g. diagnostics, and usually do not evaluate new medicines. After a new medicine has been approved by the European Medicines Agency (EMA), CIPM starts the HTA process.18 The manufacturer submits a dossier containing technical information, the price the manufacturer is asking for, expected sales, cost-effectiveness studies, the market price in other European countries, and information about the company's research and manufacturing base in Spain and whether the sale of the product will benefit Spain's economy.3,22 To inform the P&R decision, AEMPS usually produces a clinical HTA report (Therapeutic Positioning Report, IPT) about relative efficacy and safety, the nature of the disease, other therapeutic options, whether any especially vulnerable groups may benefit from the treatment, and other social or medical aspects. The IPT includes little consideration of costs,23 mainly because is made before setting the price of the medicine.
Spain operates a dual pricing system for hospital medicines. The official (list) price is for medicines for sale outside the NHS (parallel exporters and private patients). The Ministry of Health (MoH) may also negotiate a discounted and confidential “reimbursed price” for the NHS. Once the MoH and manufacturer have negotiated a price, the CIPM make the final decision on P&R. By law,24 the decision should take into account the following criteria, although none have been operationalized: a) severity of the disease; b) the specific needs of certain groups of people; c) the therapeutic and social value of the medicine and incremental clinical benefit taking into account its cost-effectiveness; d) the rational use of public expenditure and the budget impact to the health service; e) the existence of therapeutic alternatives at lower price; and f) the degree of innovation of the medicine. After a medicine has been received national P&R approval, the regions sometimes add additional restrictions. Hospitals have more discretion and may conduct local HTA.25
GermanyOnce the medicine has been approved by the EMA, the manufacturer may introduce the product into the German market at any initial price of its choosing, fully reimbursed by all German insurance plans for the first 12 months.21,26,27 During this time, the Federal Joint Committee (G-BA), can request a clinical HTA from the Institute of Quality and Efficiency in Healthcare (IQWiG). Manufacturers submit a dossier to IQWiG. Informed by IQWiG's recommendations, the G-BA determines the new medicine's added benefit over the best current therapy, on a six point scale:
- 1.
Major added benefit — sustained and substantial improvement not previously achieved by current therapies (this usually requires proof of substantially improved overall survival).
- 2.
Considerable added benefit.
- 3.
Minor added benefit.
- 4.
Added benefit present but not quantifiable.
- 5.
No added benefit proven.
- 6.
Lower benefit than current therapies.
In addition, IQWIG evaluates the quality of the evidence, reported in three categories: proof of benefit, indication of benefit, hint of benefit.
If the G-BA ranks the added benefit in any of categories 1, 2 or 3, then the manufacturer can negotiate the price with public health insurance providers. If parties cannot reach agreement, the matter is submitted to arbitration. If a medicine offers no additional value over a previously available medicine (categories 4, 5 and 6) then payers will reimburse only at prices currently paid for the older existing medicines. Pharmaceutical companies can choose to sell their product at higher prices, though patients who want the newer and lower ranked medicine must pay the difference out of their own pockets. If a manufacturer charged an excessive rate for a lower ranked medicine in the first year, the extra revenues must be returned to the health insurers. A pharmaceutical company can opt for their medicine to not be assessed, in which case a medicine's price is based on that of others in the same therapeutic class, including generic alternatives. IQWiG initially proposed a methodology for economic evaluation based on the “efficiency frontier”,28 but this is now mainly used only where price negotiations have failed.10
FranceHTA is carried out by the French National Authority for Health (HAS) for all new medicines.20 HAS produces a structured opinion within 90 days on: 1) clinical benefit and therapeutic interest (SMR); 2) level of improvement of clinical benefit (ASMR); or 3) incremental cost-effectiveness ratio. Cost-effectiveness analysis in France is not conducted for all medicines, but only if the manufacturer solicits recognition of at least a significant improvement in clinical benefit (ASMR grade I-III) or the product or technology is likely to have a significant impact on health insurance expenses (> €20 million in annual sales during the second full year) or an impact on the organization of care, professional practices or patient care. The HTA process can be single technology appraisal (the new medicine is compared against a single comparator) or multiple technology appraisal (all therapeutic options are compared for that patient group). HAS undertaken a review of the decision every 5 years or earlier if new evidence becomes available.
The SMR rates the product in one of four categories: important, moderate, mild or insufficient. The rating is based on the criteria of efficacy and safety, the severity of the disease, the position of the medicine in the therapeutic strategy and the existence or absence of therapeutic alternatives, the type of treatment (preventive, curative or symptomatic), and the public health impact. Of these, the first two criteria seem to be the most decisive.29 For outpatient medicines, the National Organisation of Health Insurance Funds decides the reimbursement level according to a sliding scale.30 Patients pay the full price in the pharmacy and claim back reimbursement from their health insurer. A SMR rating of “important” benefit gives place to a reimbursement rate of 65%, a SMR rating of “moderate” benefit gives a reimbursement of 30% and an SMR rating of “mild” benefit gives reimbursement of 15%. Medicines with an “insufficient” benefit rating will not be included in the positive list. For inpatient medicines, the MoH decides if it will be reimbursed or not, based on the SMR. If it is included in the positive list, it will be reimbursed at 100%. The final decision about whether the medicine will be used is taken at local level by each hospital pharmacy committee.
HAS also rates the new medicine according to five ASMR levels, from I —major innovation (a demonstrated effect on mortality in a severe disease)— to V —no improvement, compared with existing therapies—. Unless the product is first to market in its class, the evaluation is done in comparison with enlisted products of the same therapeutic class. The Economic Committee for Health Products decides the ex-factory price based on the ASMR rating. Medicines rated ASMR V can be listed only if the costs are less than the comparators. Medicines rated ASMR I-IV can have the possibility of a higher price than the comparator. Medicines rated ASMR I-III can obtain faster access to market (price notification instead of negotiation) and price consistency with European ones. A department of HAS evaluates the manufacturer's cost-effectiveness report.31 As France does not use an explicit cost-effectiveness threshold, it is difficult to say how the cost-effectiveness analysis influences price negotiations,32,33 although HAS has rejected some manufacturers’ analyses because of methodological concerns.34
SwedenThe Dental and Pharmaceutical Benefits Agency (TLV) has direct decision-making power over outpatient prescription medicines, whereas the 21 county councils are independently responsible for hospital/in-patient medicines. P&R are decided simultaneously based on the evidence provided by the manufacturers.19 The TLV uses three broad criteria: 1) human value; 2) need and solidarity; and 3) cost-effectiveness.
The first of these is a general ethical principle, the equal value of all human life. The second is operationalized in terms of disease severity, i.e. more severe conditions should be given a higher priority, while the third uses cost per quality-adjusted life-year (QALY) from a societal perspective.35 There is no explicit threshold, but examination of decisions made suggests the half of new medicines accepted in the period 2005-2011 had incremental cost effectiveness ratios less than €79,400/QALY for non-severe diseases and €111,700 for severe diseases.35 Budget impact is not a formal criterion.36
The medicine manufacturer proposes a price and the TLV accepts or rejects the application. TLV consults with the manufacturer before making the decision public. The manufacturer can choose to withdraw the application at this stage and the information in the application in this case will not be published.14 Prices are not negotiated, but if a medicine was effective, but was rejected because the cost-per-QALY was too high, the manufacturer can resubmit with a lower price.37
EnglandEngland has free pricing, though moderated by profit control.17 Provided the medicine is not on a negative list, NHS professionals can prescribe the medicine as soon as it receives marketing license, and the NHS reimburses medicines at 100% of the price (apart from a statutory co-payment for medicines purchased in community pharmacies).
The National Institute for Health and Care Excellence (NICE) selects medicines and other health technologies for HTA where national guidance is expected to add value (clinical impact, variation in practice, need for information or resource impact), and aims to appraise all new inpatient and ambulatory medicines and indications.38 These evaluations can be initiated when NICE receives notification that the company is applying for regulatory approval (horizon scanning), but if NICE is not tracking a regulatory submission, the process may be initiated after launch. The process and timetable depend on whether the appraisal is single technology (STA) or multiple technologies (MTA). An STA will take at least 32 weeks to complete from initiation to publication of guidance. An MTA can take at least twice as long. To speed up the process further, NICE in 2017 introduced the fast-track option for appraising medicines which offer exceptional value for money, aiming for them to become available a month after receiving marketing authorization.39 NICE bases decisions on cost-utility analysis and has an explicit ICER threshold between £20,000 and £30,000 per QALY, with flexibility to recommend medicines for patients at the end of their lives or for very rare diseases at a higher ICER.40
Local payers must, by law, finance technologies approved by NICE. To speed up access, NICE applies a “budget impact test”. Medicines that are forecast to cost more than £20m in any one of their first three years of use will trigger commercial discussions between the company and NHS England to mitigate the impact on the rest of the NHS.39
Following an unfavourable decision by NICE, payers are not obliged to finance the medicine. The manufacturers in some cases enter into negotiations with the Ministry of Health over “patient access schemes” to try to improve its cost-effectiveness. Such a scheme might contemplate a price discount, overall expenditure cap, or risk —sharing agreement—. Some medicines rejected by NICE have in the past been publicly financed by other means (e.g. Cancer Fund).
DiscussionHistorically, Spain has been slow to implement the legal and institutional measures to formalise HTA, with the result that, in comparison with other countries, the P&R process lacks clear criteria and allows excessive discretionality.2,3 Spain was one of the first countries to set methods guidelines for economic evaluation within HTA, but these were never officially adopted.41 Nevertheless, considerable progress has been made in recent years since the establishment of the clinical HTA reports (IPT) in 2013.42 We make specific recommendations for further development with the objective of improving transparency, governance, economic evaluation and efficiency (summarized in Table 4). In each case, the recommendation is justified by comparison with other countries and the expected benefit arising from implementing the change. We have aimed to be objective and constructive in our criticisms, though of course the recommendations are grounded in the knowledge and experience of the authors.
Transparency allows public scrutiny and democratic oversight of technocratic decisions. Drummond et al. recommend that the HTA process is conducted independently of the body that will ultimately be responsible for adopting, paying and implementing the decision (the health insurance funds or national health service).43 The HTA bodies in England, Sweden and Germany have greater legal autonomy than France and Spain, where the institutions are agencies of the MoH. Spain is the only country among those reviewed in this paper where the P&R decision is made by a body that includes appointees from outside Health, that is, the ministries of Finance, Industry and Economy. Reference is made in the documentation to the “contribution of the manufacturer to gross domestic product”,22 but the meaning of this concept is vague. As there is little transparency about how P&R decisions are actually made, it cannot be ruled out that favourable terms are being given to companies with a manufacturing or research base in Spain. This practice may contravene European competition law, as well as being against the interests of Spanish consumers and tax-payers.3 Governance could be improved by strengthening the independence of the bodies charged with producing supporting HTA data, freeing decision-making bodies from political influence,44 and separating healthcare decisions from industrial policy.
Transparency is far from perfect in other countries. HAS, NICE and IQWIG/G-BA publish most of the technical reports used to make decisions. Nevertheless, “commercially confidential” information is censored. The TLV only publish a “summary of decision”, and gives companies the opportunity to withdraw rather than publish a negative decision. AEMPS in Spain publishes the HTA report (IPT) but do not publish other documentation used by the CIPM to make the decision. After the decision is made, the IPT is amended to state whether the medicine is financed or not in the NHS. However, specific reasons are not usually elaborated. A more transparent process would relate the decision more closely with the evidence.
While all countries publish the official price, they often negotiate confidential discounts. Pharmaceutical firms ask for transparent P&R criteria in order to correctly align their long term investment decisions with payers’ priorities.45 But at the same time, manufacturers ask for discounts to remain confidential, because many countries set prices for medicines by comparing against the official prices paid in other countries (international reference pricing). Therefore Spain is not an “outlier” in withholding this information. The lack of transparency makes it difficult to assess whether value for money is being achieved in public procurement.
Other countries are more transparent than Spain in procedures, decision making criteria and the involvement of stakeholders. Procedures and documentation could be made clearer. The reasons for decisions and areas of uncertainty should be published. Stakeholders, patients and the public should be encouraged to participate and be consulted on draft versions of the decisions. NICE has made considerable progress in involving stakeholders and might be considered a reference.7
There are currently no formal criteria for linking price to therapeutic value in Spain. Other countries use formal scales for defining health gain relative to a comparator (ASMR in France, Added Benefit in Germany, QALY gain in Sweden and England), and to a greater or lesser extent, the price of the medicine is related to this measure of health gain. The clinical HTA reports (IPT) produced by the agencies in Spain are of a high methodological quality, but the health gain is not mapped to a generic scale as elsewhere. The use of such a measure would improve efficiency and transparency of pricing, by relating the price to health benefit in a more consistent way across different pathologies and indications.
The law in Spain requires cost-effectiveness analysis to be taken into account, but does not say how this should be applied. This ambiguity about the role of economic evaluation is not unique to Spain; France requests cost-effectiveness analysis, but it is unclear how this impacts on the decision. German decision makers appear reluctant to use it, apparently based on ethical and legal concerns about “monetizing” health.27 Guidelines for hospital formularies in Spain recommend a fairly simple cost-effectiveness and budget impact methodology.25 Academic papers propose more sophisticated methods,46 some recommending an explicit cost-per-QALY threshold.5,47–49 It is recommended in health economic analyses to use a long time horizon, and incorporate real-world effectiveness data, rather than only short-term efficacy. However, there may be a trade-off between ensuring rapid access to new medicines and having the time to undertake sophisticated modeling.
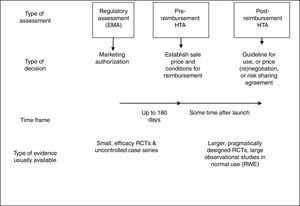
All countries aim to make prompt decisions to ensure timely access to new medicines. Nevertheless, there are important differences between countries in the purpose of HTA, and this influences what type of HTA is most appropriate. In France and Sweden, as in Spain, the price at which any medicine can be sold must be determined by the relevant national authority before the medicine can be marketed. The 180 day timetable set by the EC directive aims to ensure prompt access, but may also put practical limits on the extent of analysis and modelling that can be undertaken (Fig. 1). To some extent, the free-pricing model followed by England and Germany allows immediate access by patients to new medicines in these countries, while allowing time for HTA agencies to collect more data and conduct a thorough set of analyses. However, in practice providers in England are reluctant to prescribe before NICE has issued guidance, and NICE has been under pressure to speed up decision making.50
All the countries in this review ask the manufacturer to submit a dossier of evidence. In Sweden and Germany the HTA agencies review and comment on the manufacturer's dossier rather than conduct new analyses in-house, and this is increasingly the case for P&R in England as well. The advantage of this approach might be quicker P&R decisions and less cost for the HTA agency, while the limitations are that decisions are made in an incremental, piecewise manner as each new product comes on to the market (rather than compare all options head-to-head), and perhaps allows the manufacturer greater discretion about what to include in the dossier or how to interpret this data. The risk of conflict of interest is particularly acute in the case of economic evaluation which requires assumptions and judgments about what to include in the model. If manufacturers are charged with preparing the clinical and economic evidence, then the decision maker must be prepared to reject a manufacturer's submission (and hence, possibly, reject the application for P&R) if it falls short of the required methodological rigour. A high level of transparency is needed to ensure proper democratic oversight. In any event, Spain needs to invest in sufficient researchers with the necessary skills to review these clinical and economic evaluations and to strengthen substantially the technical staff supporting the national P&R committee. It is noteworthy that currently the regional HTA agencies (AETS) produce and publish evaluations of a wide range of healthcare programs (mainly devices and diagnostic technologies),51 but play almost no part in the P&R process for new medicines. Agencies in other countries conduct HTA for all types of therapies and technologies, in accordance with recommendations for best practice.43
AEMPS conducts clinical HTA, but does not evaluate costs or cost-effectiveness. One reason given for this is that the price is unknown, as the clinical HTA report precedes the P&R decision. However, this does not have to be an obstacle. HTA agencies in Sweden, France and England conduct or review cost-effectiveness analyses using the price the manufacturer is asking for. Another reason given is that discussion of cost is outside the remit of AEMPS. We recommend that full HTA of costs and benefits of new medicines should be coordinated within the regional Network, supported by AEMPS.
HTA needs to be appropriate for the decentralized Spanish health system. An important element of P&R process is whether the decisions are advisory or binding on the health service. In Spain, although the CIPM makes a central P&R decision, the 17 regions and hospitals exercise some discretion, particularly for inpatient medicines. Hence the nationally set price in Spain is the “maximum price”, and a positive reimbursement decision at national level is necessary but not sufficient to ensure access for all patients.
If regions and local hospitals are making independent decisions, then this should avoid duplicating technical tasks (for example, systematic review or model construction). With this in mind, coordination among the different HTA agencies in Spain (including AEMPS and AETS) might be strengthened. More research is needed about the impact of decentralized decision making on inequality in access.52
Editor in chargeClara Bermúdez-Tamayo.
Various studies have compared health technology evaluation (HTA) in Spain with other countries, and have recommended greater transparency and independence among the HTA agencies.
What does this study add to the literature?This is the first study that has Spain with other countries specifically looking at processes and criteria for pricing and reembolsement of new medicines.
The corresponding author on behalf of the other authors guarantee the accuracy, transparency and honesty of the data and information contained in the study, that no relevant information has been omitted and that all discrepancies between authors have been adequately resolved and described.
Authorship contributionsD.E. conceived and designed the study. Both authors reviewed the literature, analysed the data, drafted the manuscript and authorised the final version.
The authors wish to thank Félix Lobo, Erica Visintin and Anna-Maria Fontrier for valuable comments.