To determine the effectiveness of a brief intervention in increasing influenza vaccination coverage compared with the usual advice in people who refuse it, and to record the main reasons for refusing to be vaccinated.
MethodA cluster randomized clinical trial was conducted in which the study population was individuals with high risk factors who initially had refused to be vaccinated against influenza. Professionals (doctors and nurses) who voluntarily accepted to participate were assigned randomly to the intervention group (brief intervention) and the control group (usual advice).
Results57 professionals recruited 524 people who had previously declined the influenza vaccination (271 in the control group and 253 in the intervention group). Brief intervention showed its effectiveness with an odds ratio of 2.48 (1.61-3.82; p<0.001), in individuals aged 60 or over, both healthy or with risk factors. The most frequent reasons for rejection of vaccination were the belief that there was no risk of getting sick (53.0%) and the fear of the side effects (33.3%).
ConclusionsBrief intervention is an effective tool in improving vaccination coverage in people who have initially rejected it.
Determinar la efectividad del consejo breve para la vacunación antigripal frente al consejo habitual en personas que la rechazan, y conocer los principales motivos de rechazo.
MétodoSe realizó un ensayo clínico aleatorizado por clusters, en el que la población de estudio eran personas con factores de riesgo y que inicialmente rechazaban vacunarse. Los/las profesionales (médicos/as y enfermeros/as) que aceptaron participar se distribuyeron aleatoriamente en un grupo de intervención (consejo breve) y un grupo de control (consejo habitual).
ResultadosCincuenta y siete profesionales reclutaron 524 personas que rechazaron la vacunación (271 en el grupo control y 253 en el grupo de intervención). El consejo breve demostró su efectividad, con una odds ratio de 2,48 (1,61-3,82; p<0.001), en las personas de 60 años o más, sanos o con factores de riesgo. Los principales motivos para no vacunarse fueron la creencia de no estar en riesgo de enfermar (53,0%) y el miedo a los efectos secundarios (33,3%).
ConclusionesEl consejo breve es una herramienta efectiva para mejorar las coberturas de vacunación en personas que la rechazan inicialmente.
Influenza is considered to be a serious public health problem, since it causes high morbidity rates during annual epidemics (between 5 and 20% in the general population).1 In addition, it can more severely affect people belonging to certain high-risk groups (such as those aged over 60, people with chronic conditions, pregnant women).1 World Health Organization has recently estimated that every year 290,000 to 650,000 deaths are associated with respiratory diseases from seasonal influenza.2 In the region were the present study was carried out (Catalonia, Spain) mortality rates were 13.4% according to data from the 2017-2018 season, similar to previous years.3
The inactivated influenza vaccine (IIV) is, alongside with hygiene measures, the main prevention strategy against influenza and its complications.1 The vaccine immune response is variable: in young and healthy people its effectiveness can be between 70 and 90% to prevent influenza. Between 30 and 70% of the hospitalization for influenza and pneumonia can be prevented by the IIV. In the elderly in institutional care, the classic inactivated virus vaccines have shown to have an effectiveness of between 50 and 60% to prevent hospitalization and pneumonia and 80% in preventing death from influenza.1
Although IIV recommendation is widely practiced and accepted, vaccination coverage has remained relatively low. According to the Spanish Ministry of Health, IIV rates for people aged over 65 during the 2016- 2017 campaign stood at 55.5% for Spain as a whole and 54.3% in Catalonia.4
Some studies in adults5,6 have explored patients and doctors’ reasons for deciding whether or not to get vaccinated against the influenza virus. These studies suggest that non-vaccinating patients mention the fear of adverse side effects, a fear of contracting the illness, a belief that they do not belong to a risk group or simply having never considered it. They mention doubts regarding efficacy and safety to a lesser extent.
According to Picazo et al.7 and Giese et al.6, the role of healthcare professionals is essential in increasing IIV coverage by providing patients over 18 with information and advice. Their opinion and example may influence patients’ decisions one way or another. Certain beliefs and attitudes held by healthcare personnel influence their own vaccination rates and can prevent patients from receiving suitable health education.7
The majority of patients who have been vaccinated have done so following the advice recommendation of their general practitioners, a specialist or a nurse. These patients include both adults over 607 and children or adolescents under 18.8 Patients typically voice their doubts regarding the vaccine and its potential side effects in the nursing or doctor's consulting room. 47% of patients who have not been vaccinated would be willing to do so if its necessity was explained to them thoroughly enough.7
Numerous strategies have been tried in an attempt to promote vaccinations and increase coverage: text messages containing advice, reminders, telephone calls, and interventions such as brief education.6–9 According to a review by the European Centre for Disease Prevention and Control (ECDC),9 some of these strategies have not yet been evaluated, while others have shown a degree of effectiveness, especially in changing attitudes and beliefs regarding vaccination.
Brief intervention is a method involving the delivery of health education in a direct, personalized, short way (lasting between 3 and 15minutes),10–13 which is systematic, universal, structured and integrated into daily clinical practice. In the case of the influenza vaccination, this is an opportunistic recommendation from the healthcare professional to patients in high-risk groups. brief intervention is effective in increasing vaccination coverage rates for various vaccines and different population groups14-18: influenza vaccine in pregnant women or adults with chronic diseases, anti-pneumococcal in seniors and high risk groups, human papillomavirus vaccine in young males, among others.
Therefore, during the 2016-2017 vaccination campaign, a previous pilot study19 was carried out at an urban health centre to evaluate the effectiveness of a brief, structured, standardized education intervention which directly addressed the reasons for rejection expressed by patients. This study proved to be effective, although the sample was small and not randomized, so it was recommended to carry out a larger and randomized study.
The general objective was to determine the effectiveness of the use of brief intervention for the inactivated influenza vaccine compared to the usual advice, in people who refuse to be vaccinated.
Specific objectives:
- •
To examine the effectiveness of brief intervention compared to the usual advice in different risk groups:>60 years old healthy,>60 years old with a risk factors, and<60 years old with risk factors).
- •
Quantify influenza vaccine coverage in initially reluctant patients according to the most common risk factors.
- •
Record the patients’ reasons for refusing to be vaccinated.
The reference population consisted of patients assigned to and treated by urban and rural health centres in the centre of Catalonia, an area with a population of approximately 405,000. 135,648 were the risk factor population that could be vaccinated against influenza virus.
The study population consisted of individuals in healthcare centres during the 2017 influenza campaign. They were members of risk groups for IIV (older than 60 and healthy, under and over 60 and at risk of further complications resulting from influenza).2
Inclusion criteria:
- •
Individuals in high-risk groups for influenza (paediatric and adult, both genders).
- •
Not intending to be vaccinated against the influenza virus during the current season.
- •
Informed consent to participate. In case of paediatric patients, parents signed the consent and made decisions about vaccination.
Exclusion criteria:
- •
Language barrier.
- •
Mental or physical conditions which make it difficult for the patient or their relatives to make decisions.
- •
Having previously participated in the pilot study.
The Research Ethics Committee of the Institut Universitari d’Investigació en Atenció Primària (IDIAP Jordi Gol) approved the study protocol (P17/224).
After an email inviting all professionals in the area (905 from 32 primary care centers) to participate in the study, they were able to voluntarily decide whether or not to participate. The participants (doctors and nurses) from the different centres were randomly assigned by clusters to either the intervention group (IG) or the control group (CG) made up of 27 and 30 professionals, respectively. It was taken into account that if a doctor and a nurse who participated, were working as a team with the same assigned patients, they both participated in the same control or intervention group. All professionals who participated attended a general meeting where the study was explained; then specific instructions were given to the professionals in the CG, and finally, and only in the presence of the professionals from the IG, the brief intervention was explained in detail.
The recruitment of patients suitable to participate in the study was carried out during the IIV campaign, as part of the health centre's routine activities. Patients with inclusion criteria who came to see a doctor or nurse were invited to participate in the study. Those who accepted participating signed an informed consent.
In the IG, patients were asked about the reasons to reject the IIV and they were recorded on an Excel document. The intervention consisted of a standardized brief intervention, which varied depending on the reason the patient had given for refusing the vaccination. The advice was created following a literature search for articles, which included patient surveys to determine reasons for rejection of IIV or similar vaccines. The main reasons for rejection were divided into groups and the arguments against them were based on scientific data in order to rebut said arguments and encourage a favourable and more informed decision. Brief intervention was performed by the healthcare professional during the consultation. It was given verbally, with written support (Table 1). The reasons for non-vaccination were only collected in the IG, because it was necessary for the intervention.
Brief intervention regarding the inactivated influenza vaccine.
| 1) Ask about their intention to vaccinate against the flu. | |
| 2) Inform them of the importance of a flu vaccination in light of the patient's state of health. | |
| 3) If they refuse the vaccination, enquire as to the reasons for rejection. | |
| Reasons for refusing the IIV | Arguments in favour of IIV |
| “It has side effects”, “In previous years, I’ve caught a cold after having a vaccination” | Vaccine obtained from dead virus, it's not possible for it to cause the flu.It may produce localised swelling (15-20%) and fever or mild discomfort (2%).During the IIV period other viruses are active and the vaccine does not protect against them (colds, pharyngitis, etc.) |
| “They say that the vaccine isn’t very effective” | The vaccine is the best form of prevention available.Its effectiveness varies between 30-70%, due to the different mutations of the virus which are in circulation, but it is a means of avoiding complications. |
| “I never get sick”, “I’ve never had the flu” | Every year, 5-20% of the population suffers from flu. If you are in a high-risk group, a vaccination is recommended to avoid complications. |
| “The flu isn’t a serious illness” | Influenza can be a serious illness.Every year, between 300,000 and 500,000 people die due to the flu worldwide.The elderly and those with chronic illnesses have a higher risk of infection. |
| “I’m pregnant. The vaccine might have negative side effects on the foetus” | Pregnant women are more susceptible to catching the flu and at a greater risk of suffering from complications, such as giving birth prematurely.The vaccine protects the mother and also the baby for the first 6 months. |
| “They (the pharmaceutical industry) do it to make money” | Making vaccines isn’t a profitable business. Vaccines are very costly to develop and they undergo a lengthy safety and evaluation process and are intended for a small proportion of the population. |
IIV: inactivated influenza vaccine.
In the CG the IIV advice didn’t change: it was the normal advice that professionals used to give their patients, that is, any unstructured advice on IIV performed by the health professional, that they do every year during influenza vaccination campaigns. Patients in the CG were not asked for the reasons for the rejection of the vaccine to prevent them from influencing the advice.
There were no known differences between the advice given by a doctor or a nurse: in the CG was used the usual advice, and in the IG the brief intervention. Groups were selected including both types of professionals in both groups to avoid differences between groups.
Data was collected anonymously and confidentially via the electronic health record of Catalonia [eCAP in Catalan]. The variables analysed for the two groups were: IIV at the end of the 2017 vaccination campaign (yes/no), age, IIV risk factors and reasons for non-vaccination.
The SPSS v18 statistical program was used to analyse the data. IIV coverage was recorded for the CG and IG as a whole and for each risk group. The analysis of any statistical differences between the control and intervention groups was conducted using the chi-square test, with a p value<0.05 considered statistically significant.
Results57 professionals from 18 different primary care centers voluntarily participated in this study.
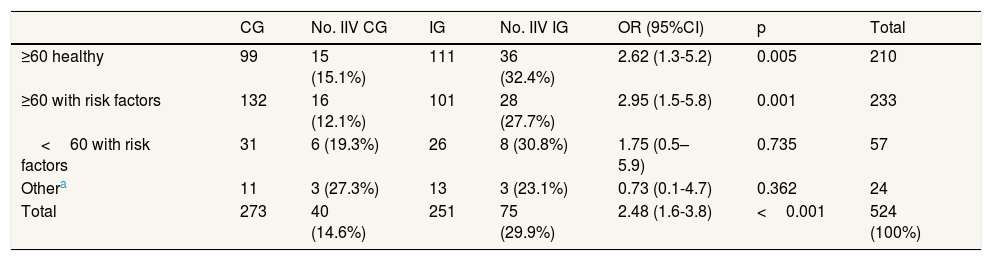
Of a sample of 524 patients belonging to a high-risk group for IIV who did not intend to be vaccinated, 251 belonged to the IG and 273 to the CG.
Of the sample obtained, 40.1% (n=210) of the patients were 60 or older and healthy, 44.5% (n=233) were 60 or over 60 with some risk factors and 10.9% (n=57) younger than 60 with risk factors (1.3% were paediatric patients, n=7). 4.6% of patients (n=24) were at risk of IIV but with risk factors, which did not appear on the eCAP list (Table 2).
Sample distribution, vaccination rates and effectiveness of brief intervention.
| CG | No. IIV CG | IG | No. IIV IG | OR (95%CI) | p | Total | |
|---|---|---|---|---|---|---|---|
| ≥60 healthy | 99 | 15 (15.1%) | 111 | 36 (32.4%) | 2.62 (1.3-5.2) | 0.005 | 210 |
| ≥60 with risk factors | 132 | 16 (12.1%) | 101 | 28 (27.7%) | 2.95 (1.5-5.8) | 0.001 | 233 |
| <60 with risk factors | 31 | 6 (19.3%) | 26 | 8 (30.8%) | 1.75 (0.5–5.9) | 0.735 | 57 |
| Othera | 11 | 3 (27.3%) | 13 | 3 (23.1%) | 0.73 (0.1-4.7) | 0.362 | 24 |
| Total | 273 | 40 (14.6%) | 251 | 75 (29.9%) | 2.48 (1.6-3.8) | <0.001 | 524 (100%) |
CG: control group; 95%CI: 95% confidence interval; IG: intervention group; IIV: inactivated influenza vaccine; OR: odds ratio.
Vaccination coverage in these patients (who had rejected the IIV, but who were part of a high-risk group) was 21.9% (n=115): 24.3% of those 60 or over 60 who were healthy, 18.9% of patients 60 or over 60 with various risk factors and 24.6% of those under the age of 60 with risk factors.
The main risk factors of patients who initially rejected the vaccine but were finally vaccinated were metabolic (25.4%), kidney (17.9%), cardiovascular (17.4%) or pulmonary (13.0%) disease (Table 3). There were no statistically significant differences in the vaccination according to which one of the risk factors.
Influenza vaccination and risk factors.
| Anti influenza vaccination 2017 | Total | Statistical hypothesis testing | p | ||
|---|---|---|---|---|---|
| Yes | No | ||||
| IIV risk factors | n=115 | n=409 | n=524 | ||
| Renal insufficiency | 20 (21.3%) | 74 (78.7%) | 94 (17.9%) | 0.03 | 0.862 |
| Cardiovascular disease | 20 (21.9%) | 71 (78.1%) | 91 (17.4%) | 0 | 0.994 |
| Metabolic syndrome | 24 (18.1%) | 109 (81.9%) | 133 (25.4%) | 1.584 | 0.208 |
| Lung disease | 13 (11.3%) | 55 (80.8%) | 68 (13.0%) | 0.365 | 0.546 |
| Other factors | 10 (37.0%) | 17 (63.0%) | 27 (5.1%) | ... | ... |
IIV: inactivated influenza vaccine.
The main reasons for not getting vaccinated, in both age groups, were “I never get sick” (53.0%) and “It has side effects” (33.3%). The least frequent motive was “They (the pharmaceutical industry) do it for the money” (0.4%) (Table 4). 17 results were lost due to incorrect registration.
Rate of influenza vaccination and reasons for rejection of vaccination.
| Influenza vaccination 2017 | |||
|---|---|---|---|
| Yes | No | Total | |
| Reasons for refusal | n=71 | n=163 | n=234 |
| Fear of side effects | 25 (32.1%) | 53 (67.9%) | 78 (33.3%) |
| The vaccine is not effective | 8 (40.0%) | 12 (60.0%) | 20 (8.6%) |
| I never get sick | 34 (27.4%) | 90 (72.6%) | 124 (53.0%) |
| It's a minor illness | 1 (33.3%) | 2 (66.6%) | 3 (1.3%) |
| They (the pharmaceutical industry) do it to make money | 0 (0.0%) | 1 (100%) | 1 (0.4%) |
| Other | 3 (37.5%) | 5 (62.5%) | 8 (3.4%) |
At the end of the IIV campaign, 75 patients (29.9%) in the IG were vaccinated and 40 in the CG (14.6%). The intervention was effective at the global level (odds ratio [OR]: 2.48 [1.61-3.82]; p<0.001) and in those aged 60 and over 60 (healthy OR: 2.62 [1.32-5.17], and with risk factors OR: 2.95 [1.49- 5.79]). There were no statistically significant differences in the effectiveness of the intervention in those aged under 60 with risk factors or between individuals with different illnesses (Table 2).
DiscussionThe results obtained in this study show that IIV coverage in patients with vaccine hesitancy continues to remain low, in all groups. In order to increase vaccination coverage, there is a clear need to develop measures aimed at achieving changes in attitudes and certain behaviours,13 in the same way other behaviours which have a negative impact on health are addressed.
The present study raises the possibility of improving the coverage of IIV with a low-cost intervention, which is easily incorporated into a primary healthcare professional's routine, by means of brief intervention lasting 2-5minutes, something that is practicable on a daily basis.
Our study suggests that a structured brief intervention in influenza vaccine, as in studies conducted with other vaccines,12–16 is very effective in improving IIV rates, especially in those patients aged over 60, regardless of whether they are in a high-risk group for contracting influenza.
According to Nyhan and Reifler20, and the ECDC9, overcoming myths and mistaken beliefs in relation to vaccines may not be sufficient to improve immunization rates. In the present study, brief intervention has been shown to be effective in individuals with vaccine hesitancy. However, it is equally important that each case is dealt with on an individual basis in order to provide them with suitable information so that individuals can make an informed decision.
The implementation of this intervention in primary care could be very useful in health management and have a big impact. This measure would be a valid and effective tool for recommending vaccination to patients with vaccine hesitancy and thus increase vaccination coverage, something that numerous organizations and health institutions declare to be a priority every flu season.1
Certainly, in some cases, using the brief intervention is not enough to get them vaccinated against the flu. For this reason it's very important to use the different tools available. There are also experiences with phone calls, reminder letters, on-going recommendations, or sending short text messages (SMS)6–9. For example, the simple reminder via SMS to the high risk population, makes it possible to increase the vaccination rate, especially in children under 5 and pregnant women. It is also recognized that the content of the SMS is important and better results are found when educational and information elements are included in the message, and not just a reminder of the need to be vaccinated.9,21
Professionals who voluntarily participated were motivated professionals for the influenza vaccination campaign and to give and to improve their advice. The use of a cluster randomised trial for the intervention/control groups eliminated a possible selection bias, which was present in the pilot study,19 thus consolidating the results obtained. To minimize a possible bias and to prevent from influencing the usual advice in the CG, the professionals in this group didn’t know the brief intervention and how to develop it. In the primary care setting, different methods are used to give advice, although they were not controlled here. However, the present study has verified the impact of the brief intervention carried out by health professionals on the vaccination rates of the intervention group.
Furthermore, perhaps there could be a convenience bias as patients were recruited as they went to the health center. In future research, possible randomization should be considered.
In comparison with the strength of similar methods assessed in a meta-analysis of 61 trials,22 the current study also reveals a high OR. But the difference with other studies is the inclusion of patients who initially rejected the vaccine.
The present study included paediatric patients in the sample, since it was decided that brief intervention could also prove effective in this group. Nevertheless, the sample of patients in this subgroup was small and they could not be analysed as an independent group.
Future research should be directed to the study of brief intervention's effectiveness in people with illnesses, which were poorly represented in the sample, such as neoplasms, hemoglobinopathies or those who were immunosuppressed. It would also be interesting to specifically explore their effectiveness in other groups who are in a high-risk group for contracting influenza, such as healthcare professionals, caregivers, pregnant women or essential workers (like police officers, fire-fighters, etc.), and to explore other methods to improve vaccination in young people with risk factors, where there is no important advantage with the brief intervention. Finally, it would also be important in future research to make an effort to train professionals before and during the brief intervention.
ConclusionsBrief intervention is an effective, low cost and easy-to-use tool for health professionals that can change attitudes, beliefs and behaviour, thus achieving an improvement in influenza vaccination coverage. Main reasons for not getting vaccinated were the perception of never getting sick and fear to side effects.
Future research ought to be directed towards evaluating the application of this measure in other population groups and illnesses.
The flu becomes a public health problem every year, but vaccination rates remain low in patients and professionals, that explain different arguments and reasons to reject vaccination. Different strategies and actions have been evaluated to improve coverage. A brief intervention has shown its effectiveness with other vaccines and subjects.
What does this study add to the literature?The brief intervention for the influenza vaccine is an effective and efficient method of structured health education in improving influenza vaccination rates. Its implementation is easy, fast and systematic, and therefore in primary care could have an important social and impact on public health and health management.
Andreu Segura.
Transparency declarationThe corresponding author on behalf of the other authors guarantee the accuracy, transparency and honesty of the data and information contained in the study, that no relevant information has been omitted and that all discrepancies between authors have been adequately resolved and described.
Authorship contributionsR. Muñoz-Miralles, C. Sant Masoliver, S. Bonvehí Nadeu, A. Martín Gallego and J. Gómez del Canto contributed to the conceptualization, study design, data collection and writing of the article. J. Mendioroz Peña contributed to the study design and data analysis. A.M. Bonet Esteve contributed to the conceptualization, study design and recruitment of professionals who participated. All of the authors reviewed and approved the article prior to its publication.
RegisterClinicalTrials.gov Identifier: NCT04568785. Available at: https://clinicaltrials.gov/ct2/show/NCT04568785
AcknowledgementsThis study has been made possible thanks to the collaboration of the primary health care doctors and nurses: Javier Aibar, Alicia Ariño, Joan Armengol, Ester Arnau, Cristina Ayala, Anna Aymerich, Marta Barrufet, Montserrat Belmonte, M. Dolors Circuns, Rosa Cirera, Montserrat Ciurana, Eva Codinach, Engràcia Atienza, Anna Crespiera, Concepció Espinós, Anna M. Ferrer, Francisca Garcia, Montse Girbau, Anna M. Guiu, Francisca Haro, Mireia Hernández, Estrella Lavado, Montserrat Llorens, Trinidad López, Montserrat Nieto, Mercè Oliva, Silvia Otin, Alexandra Plana, Noelia Reñé, Carme Rialp, Berta Rodoreda, M. Josep Rodriguez, Jordi Ros, Olivia Rubio, Dolors Saavedra, Anna M. Sagarra, Jaume Sanahuja, Consol Sánchez, Encarna Sánchez, Carme Sebastià, Fina Serra, Victoria Tarin, Joan Tobias, Judith Valecillos, Carles Valero, Ester Vila Baño, Ester Vila Marzà and Olga Villarta. We would also like to thank Montserrat Rodriguez and Pilar Puig (Althaia's Primary Health Centers) for her collaboration drawing up the brief intervention, Olga Llamazares Robles for her work and contributions to the study, and Lucia Ramos and Cristina de Vega for their logistical support.
FundingNone.
Conflicts of interestsNone.