To conduct a cost-utility analysis on an integrated healthcare model comprising an assigned internist and a hospital liaison nurse for patients with multimorbidity, compared to a conventional reactive healthcare system.
MethodsA cluster randomised clinical trial was conducted. The model consisted of a reference internist and a liaison nurse, who aimed to improve coordination and communication between levels and to enhance continuity of care after hospitalisation. We recorded sociodemographic data, diagnoses and corresponding clinical categories, functional status, use of healthcare resources and quality of life. Data were collected by reviewing electronic medical records and administering questionnaires. We performed univariate and multivariate analyses both for utilities and total costs. Bootstrapping methods were applied to calculate the confidence ellipses of incremental costs and efficiency.
ResultsWe recruited a total of 140 patients. The model assessed was not found to be efficient in general. We found an incremental cost of €1,035.90 and an incremental benefit of −0.0762 QALYs for the initiative compared to standard care after adjusting for the main variables. However, the subgroup of patients under 80 years of age with three or more clinical categories resulted in an 89% cost saving in the simulations.
ConclusionsThe integrated model was not suitable for all study patients. However, the subgroup analysis identified a narrow target population that should be analysed in future studies.
Evaluar en términos de coste-utilidad un modelo de atención integrada a pacientes pluripatológicos basado en el internista de referencia y la enfermera de enlace hospitalario, comparado con un sistema asistencial convencional reactivo por episodios.
MétodosSe realizó un ensayo clínico aleatorizado por conglomerados. La intervención se basó en un internista de referencia y una enfermera de enlace hospitalario. Ambos trabajaron en la coordinación y la comunicación entre niveles y en la mejora de la continuidad de cuidados después de un ingreso. Se recogieron datos sociodemográficos y los diagnósticos con sus correspondientes categorías clínicas, así como el estado funcional, la utilización de recursos y la calidad de vida. Se utilizaron los registros electrónicos médicos existentes y cuestionarios administrados. Se realizaron análisis univariados y multivariados tanto para las utilidades como para los costes totales. Mediante bootstrapping se calcularon las elipses de confianza de los costes incrementales y la eficiencia.
ResultadosSe incluyeron en el estudio 140 pacientes. En general, la intervención no resultó eficiente. El coste incremental de la intervención frente al modelo convencional fue de 1035,90 € y la efectividad incremental fue de −0,0762 años de vida ajustados por calidad, al ajustar los datos por las variables más relevantes. Sin embargo, el subgrupo de pacientes menores de 80 años con tres o más categorías clínicas ahorró costes en el 89% de las simulaciones.
ConclusionesLa intervención integrada no resultó adecuada para todos los pacientes objetivo; no obstante, el análisis de subgrupos permitió identificar una población objetivo más concreta que debería ser analizada en estudios futuros.
The number of patients with multimorbidity is becoming so high that if it is not properly addressed, care for them will soon become unsustainable.1,2 Two common characteristics are that their health problems cannot be cured and that their health status is progressively deteriorating.3 In order to improve their care, Yáñez-Cadena et al.4 have underlined the need to adopt a systematic approach to design programs that combine organizational strategies and self-care. The provision of care to these patients is an excellent opportunity for innovation in healthcare integration based on the current healthcare structure, avoiding fragmentation of healthcare, but without excessive structural changes.5
In January 2011, in the Basque Country (Spain), the integrated healthcare organization was established for bringing together the primary and specialized care.6 In this context, an integrated model was introduced, based around an assigned internist and a hospital liaison nurse. However, the effectiveness of multifaceted interventions for preventing the functional decline of elderly patients is still controversial.7 In a review of the literature, Smith et al.8 underlines the need to develop efficient interventions for patients with multimorbidity. To our knowledge, there are no published studies having demonstrated that they are really more efficient than usual care, and none assessing their economic impact in Spain. Generally, it is assumed that these models increase the efficiency and the quality of care provided to patients, and also that they are efficient. However, there is a lack of systematic evaluation, including the assessment of the relative costs and benefits. High quality evidence from well-designed studies is required to supporting decision making on the long-term funding of particular types of integrated care interventions.9
The objective of this study was to assess whether an integrated care model for patients with multimorbidity, based on an assigned internist and a hospital liaison nurse, is efficient compared to the current system based on episodic reactive care.
MethodsStudy designWe carried out a cost-utility analysis based on a prospective and multi-center cluster randomized trial, with two groups of patients with multimorbidity, randomized by doctor's list. Cluster randomization trials are experiments in which intact social units or clusters of individuals rather than independent individuals are randomly allocated to intervention groups. As the organizational change was naturally applied at the cluster level we applied in our study this design to avoid treatment group contamination. This approach did not incorporate blinding and therefore its results showed a lower level of evidence.10 The participating providers were the seven primary healthcare centers of the Goierri-Alto Urola health district, together with the referral hospital, Zumarraga hospital. Patients’ randomization was based on the primary care clinicians’ randomization carried out before this study started. Patients were recruited consecutively from each health center when they met three inclusion criteria: to have at least one hospitalization episode during the past year, to be classified as multimorbid patients according the criteria of the Junta de Andalucía2 and to have given written informed consent. Exclusion criteria included patient refusal to participate in the study, living in a nursing home or being on hemodialysis. A total of 140 patients were recruited, 70 in each group. The duration of the intervention period in this study was 1-year.
InterventionThe intervention was focused on the management of care for these patients. That is, we did not change the type of clinical care provided. The intervention consisted on the implementation of an integrated health care model for multimorbid patients based on improving communication between primary care and hospital professionals. Specifically, intervention group (IG) multimorbid patients were managed by the primary care team (general practitioner and nurse) with the support of a reference internist and a liaison nurse. Reference internist gave direct support in the Health Centre and ensured smooth and flexible communication with primary care doctors. Moreover, every time patients with multimorbidity went to the hospital they were seen by their assigned internist, regardless of the required service. As soon as the patient was identified as being multimorbid the liaison nurse carried out a complete assessment (clinical, functional, psychosocial and quality of life). This information was aimed to enhance continuity of care after hospitalization in coordination with primary care to avoid re-hospitalizations. Furthermore, the liaison nurse provided health education to improve self-management of each specific disease. In the control group (CG), patients received usual care corresponding to routine practice, with no strengthening of the coordination between primary and hospital-based care. The protocol was approved by the local ethics committee.
Study variablesWe collected data from medical records on the following demographic and clinical variables: age, sex, referral health center, and clinical diagnoses, as well as the corresponding clinical categories. Data about resources consumption during 12 months included hospital admissions, emergency department attendances, visits to specialists, visits to primary care doctors and nurses and diagnostic tests recorded in the Osakidetza-Basque Health Service data base. In addition, we recorded Barthel Index scores,11 as a measure of functional status regarding basic activities of daily living at baseline; and EuroQol (EQ-5D) utility scores,12 as a measure of quality of life at baseline and the end of the study period, that is, before and 1 year after implementation of the new model.
Estimation of cost and quality-adjusted life yearsWe calculated for each patient cost and quality-adjusted life years (QALYs) during the 12 months follow-up. For estimating costs, we multiplied the rates of resources use by the unit cost obtained from the Accounting Department (stay day: €414.00/day; emergency department consultation: €140.69; 24-hour health clinic consultation: €39.01; specialized consultation: €141.04; primary care consultation: €23.11; home care visit: €69.32; CT scan: €77.63; and ultrasound scan: €33.46). The cost of the intervention per attended patient (€341.68) was calculated by dividing the salary of the liaison nurse by the number of patients under her care.
For the estimation of QALYs, we considered scores on the EQ-5D questionnaire before and 1 year after introduction of the intervention. The efficiency for each period of the intervention was calculated with area under the curve analysis, assuming linear interpolation between consecutive time points and taking into account the follow-up period for all patients included.13
Statistical analysisFirst, we tested the randomness of the samples, univariate analysis was performed using the chi-squared test or Fisher's exact test to identify any socio-demographic or diagnostic variables that differed between the two groups that had been selected randomly. A significance level of 5% was set.
Second, intervention results in terms of resource consumption during the study period were described using absolute and relative frequencies as well as mean values for the intervention and control groups. The analysis of the difference between mean values was carried out using Student t-test and Chi-squared test was used for categorical data.
Third, univariate analysis was carried out to identify the variables that were associated with efficiency and total costs. As costs and QALYs are generally skewed data, nonparametric tests were used in order to compare median values for different variables categories. However, cost-utility analysis is based on the difference in mean cost and the difference in mean effect. In fact, that occurs because the mean is important from both budgetary and social perspective. Therefore, t-test or ANOVA variance analysis were also carried out with the aim of estimating significance on mean differences between variable categories. In addition, univariate generalized linear models (GLM) were also applied in order to show its results compared to the commonly used approaches.
All variables that show association in the one by one analysis were involved in the subsequent multivariate analysis. Group (IG vs. CG) variable was included in all the final models, given that the purpose of the study was to assess the effect of the intervention on the efficiency and costs, adjusting for other variables that could influence them. Baseline Barthel Index scores were not included in the multivariate analysis to avoid collinearity with utilities, as measured by EQ-5D. Multivariate analysis was carried out using both ordinary least square regressions (OLS) and GLM. Finally joint analysis of costs and efficiency was carried out using Seemingly Unrelated Regression (SUR). The same independent variables were included in the multivariate analysis both for QALYs and for total costs in order to simplify the interpretation. The estimation of the incremental cost-effectiveness ratio (ICER), which describes the additional cost of each extra QALY, was calculated as the ratio of the coefficients of the variable group in the multiple regressions of cost and efficiency.14 In a second model using SUR subgroup analysis was performed considering also the interactions of the variables age and number of clinical categories with the variable group.
Once analysis of the original data had been performed at the patient level, we analyzed the overall distribution using a non-parametric bootstrapping method given that the sample size was small. This method makes it possible to estimate the uncertainty of the final decision in different samples assuming that initial samples of both groups represented the real population. For this purpose, we carried out 1,000 simulations, in which new samples of patients were created by selecting patients (with replacement). The bootstrapping method allowed estimating the 95% confidence intervals of the intervention effects both in costs and QALYs for the analyzed subgroups and calculating the confidence ellipses for the ICER using the variance-covariance matrix estimated in the joint analysis of costs and efficiency.14,15
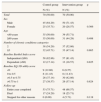
ResultsBetween April 2011 and February 2012, we recruited a total of 140 patients with multimorbidity with a mean age of 78.2 years. From them 72% completed an entire year, 25% died, and in the remaining cases (3%), follow-up was stopped as they were admitted to residential care homes. We found no significant differences between the two groups prior to the intervention as summarized in Table 1. In both groups, more than half of the patients had diagnoses in two clinical categories. Overall, 17.8% of patients obtained baseline Barthel Index scores below 60 and hence were classified as dependent.
Characteristics of the study groups at the start of the study.
| Control group | Intervention group | p | |
|---|---|---|---|
| N (%) | N (%) | ||
| Total | 70 (50.00) | 70 (50.00) | |
| Sex | |||
| Male | 45 (64.29) | 50 (71.43) | |
| Female | 25 (35.71) | 20 (28.57) | 0.366 |
| Age | |||
| <80 years | 35 (50.00) | 39 (55.71) | |
| ≥80 years | 35 (50.00) | 31 (44.29) | 0.498 |
| Number of chronic conditions categories | |||
| 2 | 38 (54.29) | 37 (52.86) | |
| ≥3 | 32 (45.71) | 33 (47.14) | 0.865 |
| Baseline Barthel Index score | |||
| Independent (≥60) | 58 (82.86) | 57 (81.43) | |
| Dependent (<60) | 12 (17.14) | 13 (18.57) | 0.825 |
| Baseline EQ-5D utility score | |||
| <0 | 9 (12.86) | 6 (8.57) | |
| 0 to 0.5 | 8 (11.43) | 8 (11.43) | |
| >0.5 to 0.75 | 26 (37.14) | 30 (42.86) | |
| >0.75 to 1 | 27 (38.57) | 26 (37.14) | 0.824 |
| Follow-up | |||
| Entire year completed | 53 (75.71) | 48 (68.57) | |
| Died | 17 (24.29) | 18 (25.71) | |
| Stopped for other reasons | 0 (0.00) | 4 (5.71) | 0.118 |
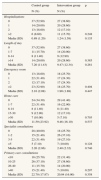
The description of resource consumption carried out by the control and intervention group is shown in Table 2. No statistical differences were found between the two groups.
Description of the main resource consumption for each group during the 1-year study period.
| Control group | Intervention group | p | |
|---|---|---|---|
| N (%) | N (%) | ||
| Hospitalizations | |||
| 0 | 37 (52.90) | 27 (38.60) | |
| 1 | 14 (20.00) | 20 (28.60) | |
| 2 | 13 (18.60) | 12 (17.10) | |
| >2 | 6 (8.60) | 11 (15.70) | 0.248 |
| Media (SD) | 0.89 (1.20) | 1.24 (1.58) | 0.135 |
| Length of stay | |||
| 0 | 37 (52.90) | 27 (38.60) | |
| 1-7 | 11 (15.70) | 14 (20.00) | |
| 8-14 | 8 (11.40) | 9 (12.90) | |
| >14 | 14 (20.00) | 20 (28.60) | 0.385 |
| Media (SD) | 7.20 (11.43) | 9.47 (12.34) | 0.261 |
| Emergency room | |||
| 0 | 13 (18.60) | 18 (25.70) | |
| 1 | 22 (31.40) | 17 (24.30) | |
| 2 | 12 (17.10) | 17 (24.30) | |
| >2 | 23 (32.90) | 18 (25.70) | 0.404 |
| Media (SD) | 2.16 (2.08) | 1.90 (1.88) | 0.447 |
| Home care | |||
| 0 | 24 (34.30) | 29 (41.40) | |
| 1-7 | 22 (31.40) | 16 (22.90) | |
| 8-14 | 8 (11.40) | 8 (11.40) | |
| 15-30 | 9 (12.90) | 12 (17.10) | |
| >30 | 7 (10.00) | 5 (7.10) | 0.703 |
| Media (SD) | 11.59 (23.92) | 9.46 (16.10) | 0.537 |
| Specialist consultations | |||
| 0 | 28 (40.00) | 18 (25.70) | |
| 1-2 | 15 (21.40) | 26 (37.10) | |
| 3-5 | 22 (31.40) | 19 (27.10) | |
| >5 | 5 (7.10) | 7 (10.00) | 0.128 |
| Media (SD) | 2.26 (2.86) | 2.40 (2.32) | 0.746 |
| Primary care consultations | |||
| <10 | 18 (25.70) | 22 (31.40) | |
| 10-25 | 26 (37.10) | 27 (38.60) | |
| 26-40 | 11 (15.70) | 14 (20.00) | |
| >40 | 15 (21.40) | 7 (10.00) | 0.297 |
| Media (SD) | 22.70 (17.87) | 20.04 (16.00) | 0.358 |
SD: standard deviation.
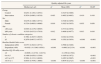
Table 3 and Table 4 show which variables had a direct impact on cost and utility of the intervention. We can see there are clear differences in QALYs by sex, age, number of clinical categories, and baseline Barthel Index and EQ-5D utility scores but none of the variables considered had an impact on the total costs when comparing mean values through usual methodology. Results are similar when univariate GLM methods were applied for mean comparison. The crude values indicate that the intervention increased the mean cost per patient by €1,093.1, compared to that for the controls. Moreover, the efficiency in patients from the intervention group was 0.0553 QALY lower.
Association between the total costs incurred in the 1-year study period and the study variables.
| Total costs | |||||
|---|---|---|---|---|---|
| Median (q1; q3) | pa | Mean (SD) | pb | GLMc | |
| Group | |||||
| Control | 2,545.8 (1,109.2; 7,553.4) | 4,973.3 (5,486.4) | |||
| Intervention | 4,492.5 (1,691.6; 9,991.7) | 0.258 | 6,066.4 (5,514.8) | 0.242 | 0.245 |
| Sex | |||||
| Male | 3,544.9 (1,432.6; 9,991.7) | 5,839.1 (5,816.8) | |||
| Female | 2,511.1 (1,316.4; 7,622.8) | 0.330 | 4,845.9 (4,783.3) | 0.321 | 0.300 |
| Age | |||||
| <80 years | 3,250.4 (1,529.7; 8,322.3) | 5,794.6 (6,005.5) | |||
| ≥80 years | 2,999.8 (1,230.9; 8,294.0) | 0.548 | 5,211.8 (4,918.7) | 0.534 | 0.529 |
| Number of chronic condition categories | |||||
| 2 | 2,580.4 (1,198.4; 7,553.4) | 4,964.0 (5,197.6) | |||
| ≥3 | 4,483.4 (1,624.4; 9,991.7) | 0.195 | 6,161.3 (5,820.6) | 0.201 | 0.203 |
| Baseline Barthel Index score | |||||
| Independent (≥60) | 2,843.1 (1,399.9; 8,564.2) | 5,527.7 (5,655.8) | |||
| Dependent (<60) | 4,662.8 (909.7; 8,294.0) | 0.884 | 5,483.8 (4,876.1) | 0.971 | 0.971 |
| Baseline EQ-5D utility score | |||||
| < 0 | 1,272.9 (369.7; 7,756.1) | 3,828.9 (3,996.1) | |||
| 0 to 0.5 | 6,615.7 (2,370.1; 9,888.2) | 6,609.9 (5,131.4) | 0.131 | ||
| >0.5 to 0.75 | 3,438.8 (1,620.0; 10,296.5) | 6,347.2 (6,073.8) | 0.084 | ||
| >0.75 to 1 | 2,197.0 (1,369.7; 6,806.4) | 0.148 | 4,795.2 (5,265.4) | 0.240 | 0.444 |
GLM: generalized linear models; q1: value for the first quartile or percentile 25; q3: value for the third quartile or percentile 75; SD: standard deviation.
Association between the efficiency in the 1-year study period and the study variables.
| Quality-adjusted life years | |||||
|---|---|---|---|---|---|
| Median (q1; q3) | pa | Mean (SD) | pb | GLMc | |
| Group | |||||
| Control | 0.6251 (0.1381; 0.8391) | 0.5235 (0.3886) | |||
| Intervention | 0.5834 (0.0861; 0.8518) | 0.493 | 0.4682 (0.4003) | 0.410 | 0.410 |
| Sex | |||||
| Male | 0.6276 (0.1486; 0.8771) | 0.5440 (0.3859) | |||
| Female | 0.5324 (0.0093; 0.6696) | 0.016 | 0.3925 (0.3955) | 0.035 | 0.050 |
| Age | |||||
| <80 years | 0.6585 (0.2179; 0.9095) | 0.5734 (0.3670) | |||
| ≥80 years | 0.5193 (0.0313; 0.7814) | 0.012 | 0.4080 (0.4077) | 0.013 | 0.016 |
| Number of chronic condition categories | |||||
| 2 | 0.7061 (0.2937; 0.8935) | 0.6162 (0.3536) | |||
| ≥3 | 0.2976 (0.0617; 0.6757) | <0.001 | 0.3592 (0.3954) | <0.001 | <0.001 |
| Baseline Barthel Index score | |||||
| Independent (≥60) | 0.6757 (0.2923; 0.8771) | 0.5996 (0.3406) | |||
| Dependent (<60) | −0.0102 (−0.1196; 0.0591) | <0.001 | −0.0002 (0.2134) | <0.001 | <0.001 |
| Baseline EQ-5D utility score | |||||
| <0 | −0.0696 (−0.2719; −0.0172) | −0.1399 (0.1504) | |||
| 0 to 0.5 | 0.1486 (0.0593; 0.3394) | 0.1958 (0.1972) | <0.001 | ||
| >0.5 to 0.75 | 0.5861 (0.1311; 0.6636) | 0.4565 (0.2695) | <0.001 | ||
| >0.75 to 1 | 0.8935 (0.7976; 1.0000) | <0.001 | 0.8076 (0.2723) | <0.001 | <0.001 |
GLM: generalized linear models; q1: value for the first quartile or percentile 25; q3: value for the third quartile or percentile 75; SD: standard deviation.
When analyzing the intervention effect adjusted by other relevant variables (sex, age, number of categories and baseline EQ5D), the results showed similar conclusions as intervention arm remained more expensive and less efficient, both using OLS and GLM (Table 5). In the joint multivariate statistical analysis (Table 5) using the simple model, we found an incremental cost of €1,035.9 and an incremental benefit of −0.0762 QALYs for the intervention compared to usual care, after adjusting for sex, patient age, number of clinical categories and EQ-5D utility score at the beginning of the study. When the interactions of the age and number of clinical categories with the variable group were included (Table 5) we could realize that considering the subgroups of patients under 80 years of age and with chronic health problems in three or more clinical categories, mean costs were lower among those in the IG but without statistical significance. Nonetheless, the benefit was also lower as was the case for all the subgroups analyzed.
Multivariate analysis of efficiency and total costs as the dependent variables.
| Cost | Efficiency | |||
|---|---|---|---|---|
| Coefficient (SD) | p | Coefficient (SD) | p | |
| Ordinary least square regression | ||||
| Intervention group | 991.87 (938.40) | 0.29 | −0.0762 (0.0398) | 0.06 |
| Female | −901.43 (1061.70) | 0.40 | 0.0627 (0.045) | 0.17 |
| 80 years old or older | −346.20 (963.38) | 0.72 | −0.0653 (0.0408) | 0.11 |
| ≥3 categories of chronic condition | 1,194.98 (997.65) | 0.23 | −0.0631 (0.0422) | 0.14 |
| Baseline EQ-5D utility score | −1.49 (1,309.02) | 1.00 | 0.7595 (0.0552) | < 0.001 |
| Constant | 4,922.93 (1,462.05) | 0.001 | 0.1296 (0.0618) | 0.038 |
| Generalized linear model | ||||
| Intervention group | 0.2372 (0.1710) | 0.17 | −0.1076 (0.0715) | 0.13 |
| Female | −0.1903 (0.1967) | 0.33 | 0.1165 (0.0842) | 0.17 |
| 80 years old or older | −0.0671 (0.1751) | 0.70 | −0.1404 (0.0729) | 0.05 |
| ≥3 categories of chronic condition | 0.2589 (0.1901) | 0.17 | −0.0676 (0.0789) | 0.39 |
| Baseline EQ-5D utility score | 0.0364 (0.2656) | 0.99 | 2.0016 (0.1959) | < 0.001 |
| Constant | 8.4504 (0.2831) | < 0.001 | −1.9685 (0.1965) | < 0.001 |
| Seemingly Unrelated Regression - Simple model | ||||
| Intervention group | 1,035.88 (925.26) | 0.26 | −0.0762 (0.0389) | 0.05 |
| Female | −836.89 (1,050.39) | 0.43 | 0.0627 (0.0442) | 0.16 |
| 80 years old or older | −310.11 (948.17) | 0.74 | −0.0653 (0.0399) | 0.1 |
| ≥3 categories of chronic condition | 1,165.43 (980.71) | 0.24 | −0.0631 (0.0412) | 0.13 |
| Baseline EQ-5D utility score | 24.03 (1,285.34) | 0.99 | 0.7595 (0.0540) | < 0.001 |
| Constant | 4,880.80 (1,437.08) | 0.1296 (0.0604) | ||
| Seemingly Unrelated Regression - Subgroup analysis | ||||
| Intervention group | 1,399.84 (1,534.25) | 0.36 | −0.0576 (0.0658) | 0.38 |
| Female | −967.26 (1,039.54) | 0.35 | 0.0594 (0.0446) | 0.18 |
| ≥80 years of age | −1,416.95 (1,326.30) | 0.28 | −0.0695 (0.0568) | 0.22 |
| ≥3 categories of chronic condition | 2,712.17 (1,351.01) | 0.05 | −0.0357 (0.0579) | 0.54 |
| Baseline EQ-5D utility score | −36.35 (1,259.94) | 0.98 | 0.7595 (0.0540) | < 0.001 |
| ≥80 years * group | 2,724.85 (1,838.22) | 0.14 | 0.0170 (0.0788) | 0.83 |
| ≥3 categories * group | −3,475.84 (1,841.26) | 0.06 | −0.0567 (0.0789) | 0.47 |
| Constant | 4,808.12 (1,583.84) | 0.1467 (0.0654) | ||
| Difference, IG vs. CG (95%CI)a | ||||
| Subgroup <80 years+2 categories | 1,399.84 (−1,704; 4,374) | −0.0576 (−0.18; 0.06) | ||
| Subgroup <80 years+≥3 categories | −2,076.00 (−5,622; 1,189) | −0.1143 (−0.27; 0.02) | ||
| Subgroup ≥80 years+2 categories | 4,124.69 (1,156; 6,980) | −0.0406 (−0.18; 0.10) | ||
| Subgroup ≥80 years+≥3 categories | 648.85 (−2,775; 3,908) | −0.0973 (−0.22; 0.03) | ||
CG: control group; IC: intervention group; SD: standard deviation.
In the cost-utility plane (Figure 1), we can see the results corresponding to the bootstrapping method applied to the original sample and the subgroup of those under 80 years old with three or more categories of chronic conditions. Considering the observed variability, we calculated the likelihood of the intervention being cost saving across the simulations performed. The subgroup of people under 80 years of age with conditions in three or more clinical categories resulted cost saving in 89% of the simulations whereas this percentage was 15% for the original sample.
DiscussionThe evaluated integrated healthcare intervention for patients with multimorbidity was not found to be efficient. However, the statistical analysis revealed that in the patients under 80 years of age with a high level of comorbidity (conditions in three or more clinical categories), the intervention decreased costs in 89% of the simulations, although the difference was not statistically significant in that subgroup either. The lack of efficiency was observed across all the subgroups analyzed which was to be expected, given the frailty and risk factors, of the target population.
Our intervention can be described in terms of the Kaiser Permanente model as a case management and our target population being included in the top 5% of the Kaiser pyramid.16,17 Regarding the subgroups analyzed, we consider that patients with multimorbidity who have only two clinical categories of conditions, regardless of age, correspond to the high-risk patients with a lower level of complexity in the Kaiser pyramid, and hence benefits would be more likely to be seen with disease management care models.
Authors that underline the importance of small changes in continuity of care for decreasing costs, resource use and complications, such as Hussey et al.,18 indicate a potential limitation of their research is that the findings might not extrapolate to younger populations, since it focused on adults over 65 years of age. In our study, we did not consider age as an exclusion criterion and conclude that the intervention could be more efficient in those under 80 years of age.
The patients aged 80 years and over with three or more clinical categories of chronic conditions correspond to the apex of the Kaiser pyramid. Besides healthcare (i.e., case management) they require coordination between social and healthcare services, giving priority to maintaining their independence as far as possible.16 Such patients do not benefit from the evaluated model as it has no impact on quality of life or disease progression, and there are more associated costs due to a higher use of healthcare resources. However, they may benefit from the use of care models for end-of-life patients.17,19
Vondeling9 suggested that the general principles of economic evaluation can be adapted to the assessment of integrated care. However, a lack of evidence on efficacy frequently hampers the economic evaluation of integrated care, contrasting with the wide availability of data on pharmacoeconomics. We based our study on a cluster randomized clinical trial providing a strong level of evidence.10,20,21 The statistical analysis enabled us to identify a subgroup in which future studies should be carried out as the intervention could be cost saving. Our results indicated that age, sex, comorbidity, and baseline Barthel Index and EQ-5D scores had an impact on the QALYs in each group, which seems logical given the level of multimorbidity, frailty and mortality in the sample. In addition to influencing perceived quality of life, in elderly people, comorbidity and disability are negative prognostic factors for functionality and survival. It should be taken into account that changes at the organization level and in healthcare management have a limited effect on the natural history of patients with high comorbidity. As a consequence, we should not expect marked changes in health outcomes.19
Literature shows that it is difficult to obtain good results in this population.22,23 However, our economic evaluation provided useful clues in terms of best suited target population to be taken into account in the implementation of future integrated programs. Moreover, it was supported by strong evidence-based design (cluster randomized clinical trial) and patient-data level statistical analysis. We have tried to solve the main three difficulties encountered by applying multivariante and subgroup analysis. First, complex patients tend to have a high level of multimorbidity. Patients with an average Charlson24 index of 5 have a 25% risk of death in the year of follow-up, masking any impact of the intervention. Second, the multifaceted nature of the healthcare model itself makes these types of intervention complex.25 Finally, we are not dealing with a clinical intervention but rather changes at the organizational level and in patient management.
This study has enabled us to assess an integrated healthcare model in terms of cost-utility. Despite the intervention not being efficient, our results are relevant as they contradict the usual assumption that integrated care models increase efficiency and quality of life, as well as leading to cost savings by decreasing hospitalizations.8,18,26 Even though this study was focused on an overall analysis of patients with multimorbidity, the subgroup analysis carried out allowed us to identify a narrow target population that should be analyzed in depth. Future research should involve studying the results of implementing the model in patients under 80 years of age with high level of comorbidity. In the light of our results, it seems clear that there is a need for more economic assessment studies to provide evidence on the efficiency of integrated models, to support their widespread use in health systems.
The increasing prevalence of chronic diseases has led to a profound change in multi-morbid patients care. Integrated programs which include new roles for nurses and physicians constitute the new paradigm but given their complex nature their implementation is challenging and must be evaluated.
What does this study add to the literature?The model assessed was not found to be cost-effective in general. However, mean healthcare costs were lower for people under 80 years of age with three or more clinical categories of chronic conditions. Integrated interventions are not suitable for all medically frail patients but rather for a subset that can be identified by statistical analysis.
Miguel Ángel Negrín Hernández.
Transparency declarationThe corresponding author on behalf of the other authors guarantee the accuracy, transparency and honesty of the data and information contained in the study, that no relevant information has been omitted and that all discrepancies between authors have been adequately resolved and described.
Authorship contributionsI. Lanzeta has participated in all the research stages (study design, clinical trial fieldwork, interpretation of the results and writing the manuscript). J. Mar has contributed to the study design and he has collaborated in the statistical analysis, in the interpretation of results and in the review of the manuscript. A. Arrospide has participated in the design of methodology and performed the statistical analysis, supported the interpretation of the results and contributed to the drafting of the manuscript. All results were discussed among the authors. All authors contributed to and have approved the final manuscript.
FundingNone.
Conflict of interestNone.
We would like to acknowledge the editorial assistance provided by Ideas Need Communicating Language Services.