To calculate, for the first time, the direct and social costs of transfusion-related adverse events in order to include them in the National Healthcare System's budget, calculation and studies. In Spain more than 1,500 patients yearly are diagnosed with such adverse events.
MethodBlood transfusion-related adverse events recorded yearly in Spanish haemovigilance reports were studied retrospectively (2010-2015). The adverse events were coded according to the classification of Diagnosis-Related Groups. The direct healthcare costs were obtained from public information sources. The productivity loss (social cost) associated with adverse events was calculated using the human capital and hedonic salary methodologies.
ResultsIn 2015, 1,588 patients had adverse events that resulted in direct health care costs (4,568,914€) and social costs due to hospitalization (200,724€). Three adverse reactions resulted in patient death (at a social cost of 1,364,805€). In total, the cost of blood transfusion-related adverse events was 6,134,443€ in Spain. For the period 2010-2015: the trends show a reduction in the total amount of transfusions (2 vs. 1.91M€; -4.4%). The number of adverse events increased (822 vs. 1,588; +93%), as well as their related direct healthcare cost (3.22 vs. 4.57M€; +42%) and the social cost of hospitalization (110 vs 200M€; +83%). Mortality costs decreased (2.65 vs. 1.36M€; -48%).
DiscussionThis is the first time that the costs of post-transfusion adverse events have been calculated in Spain. These new figures and trends should be taken into consideration in any cost-effectiveness study or trial of new surgical techniques or sanitary policies that influence blood transfusion activities.
Calcular por primera vez los costes económicos y sociales relacionados con las reacciones adversas postransfusionales para actualizar estudios e incluirlos en los presupuestos del Sistema Nacional de Salud. En España, anualmente, más de 1500 pacientes sufren dichas reacciones adversas.
MétodoSe estudiaron retrospectivamente (periodo 2010-2015) las reacciones adversas a la transfusión recopiladas anualmente en los informes nacionales de hemovigilancia. Dichas reacciones se codificaron mediante clasificación de Grupos Relacionados con el Diagnóstico. Los costes directos sanitarios se obtuvieron de fuentes públicas de información. La pérdida en productividad (coste social) asociada a las reacciones adversas se contabilizó utilizando los métodos del capital humano y salarios hedónicos, respectivamente.
ResultadosEn el año 2015, en España, 1588 pacientes tuvieron reacciones adversas que derivaron en costes sanitarios (4.568.914 €) y costes sociales debido a hospitalización (200.724 €). Tres reacciones adversas resultaron en muerte del paciente (1.364.805 €). Como suma, el coste total de las reacciones adversas a la transfusión fue de 6.134.443 €. Periodo 2010-2015: la tendencia refleja una reducción en el número total de transfusiones (2 vs. 1,91 M€; -4,4%), un incremento en el número de reacciones adversas (822 vs. 1.588; +93%), en costes sanitarios (3,22 vs. 4,57M€; +42%) y en costes sociales (110 vs. 200M€; +83%), y un descenso en costes de mortalidad (2,65 vs. 1,36M€; -48%).
DiscusiónPor primera vez se han calculado en España los costes de las reacciones adversas a la transfusión. Los nuevos datos y tendencias deberían ser considerados en estudios de coste-eficiencia sobre técnicas quirúrgicas o políticas sanitarias con repercusión en actividades de transfusión sanguínea.
Blood transfusions have shown adverse events (AEs) that augment morbidity and mortality.1 An adverse reaction or event is an undesirable response or effect in a patient, temporally associated with the administration of blood or blood component.2 Transfusion-related AEs increase morbidity and mortality and, therefore, the overall cost of their treatment. These AEs include fever, allergic reactions, viral and bacterial infections, edema and pulmonary injury, or heart damage, leading to emergency consultations, hospital admissions with diverse duration and even deaths.3
So that, the aim of this study was to assign, for the first time, a complete economic value, including social costs due to losses of productivity, of AEs attributed to blood transfusions in Spain. Secondary aim was to assess the trend in such costs during the last few years and, finally, to update the actual cost of the transfusion unit in Spain. AEs total costs’ information is crucial to update clinical trial-based cost-effectiveness analyses.
MethodsMethodology for the calculation of the health care direct overhead cost of transfusion-related adverse reactionsTo calculate the associated direct costs of the adverse reactions due to transfusion, we have matched information of three different sources. Firstly, the hemovigilance report that the Spanish Ministry of Health publishes annually.4 Secondly, the international classification of diseases (ICD) in its ninth version (ICD-9),5 and finally the DRGfinder™ program.6
We coded the diagnosis categories of post transfusion-related AEs accounted in the hemovigilance report according to the ICD-9 and then, allocated to a Diagnosis-Related Groups (DRG) by means of the DRGfinder™ program. The allocation to a given known DRG permitted to assign the direct health care costs that such AEs have for the NHS.
The DRG system is in widespread use worldwide.7,8 DRG classify patients with similar clinical grounds and similar consumption of resources into homogeneous groups and offer a structure for a precise assessment of the costs of treating a given patient.9 In Spain, the Ministry of Health, Social Services and Equality (hereinafter, the Ministry of Health) publishes annually the complete listing of DRG 10 together with costs associated to each category.
The hemovigilance report scores every AE according to clinical severity and the likelihood of being attributable to transfusion (causality). This imputability level is classified in four categories and reflects the degree of probability that the AE had been caused by the transfusion, as shown in Table 1. The hemovigilance report only takes into account and enumerates those transfusion-related AEs with an imputability level equal to or greater than 2 (likely or certain), therefore the figures expressed in the results section refer only to these episodes. In year 2014, 89% of public and 71% of private hospitals have dedicated personnel responsible of hemovigilance and 66% of all centers have a transfusion committee.
Imputability levels of adverse events due to transfusion and description in Spanish hemovigialnce report.
| Imputability level | Clasification | Description |
|---|---|---|
| 0 | Excluded or unlikely | When there is conclusive evidence beyond reasonable doubt for attributing the adverse reaction to causes other than the blood or blood components When the evidence is clearly in favour of attributing the adverse reaction to causes other than the blood or blood components |
| 1 | Possible | When the evidence is clearly in favor of attributing the adverse reaction to causes other than the blood or blood components |
| 2 | Likely | When the evidence is clearly in favor of attributing the adverse reaction to the blood or blood component |
| 3 | Certain | When there is conclusive evidence beyond reasonable doubt for attributing the adverse reaction to the blood or blood component |
Hemovigilance reports are disclosed on an annual basis, showing inter-year variation in the number of blood recipients who suffer from transfusion-related AEs, their relative prevalence and the number of recipient's deaths. Also, DRG systems need to be updated regularly to ensure that DRGs remain clinically meaningful and economically homogeneous.9
Methodology for the calculation of the social cost of transfusion-related adverse eventsTo calculate the social costs of transfusion-related AEs we classified the social costs into two categories: the cost of working days lost per day of hospital stay due to adverse reactions; and, the cost of deaths attributed to such AEs.
For the calculation of working days lost, each DRG, besides charging the rate corresponding to the clinical management, refers an average length of stay (ALOS) expressed in days upon admission, according to the information provided by all the hospitals belonging to the NHS. The human capital model 11 was used to assess the cost in terms of lost productivity, as recommended for economic evaluations.12 This method is relatively straightforward since it involves multiplying the number of lost working days by the salary of an employee who is absent. For the purpose of this study we have used a gross average income of 22,935€ in year 2015 as reported by the Spanish National Institute of Statistics.13 Daily average salary was calculated dividing the annual income by 365 days, because the number of hospitalization days referred in the DRG listing provided by the Ministry of Health does not distinguish between working and non-working days. Accordingly, the average daily salary in year 2015 in Spain resulted 62.83€ (22,935€/365 days). However, 50% of patients transfused in Spain are over 70 years and 6.4% are under 20,14 therefore only 42.6% of the transfused recipients fit into the working-age population. This latter proportion was taken into account for the calculation of the social cost of loss of productivity.
Although the frequency is very low, sometimes post-transfusion AEs can cause recipient's death. To assign a social cost to mortality we used the method of hedonic salaries evaluated by Riera et al.,15 as recommended by Viscusi,16 to allocate social costs. The last calculation of the value as a statistical life (VSL) in Spain was performed in year 2000 and was 2.37 million €. The VSL in year 2000 was updated taking into account the general consumer price index (CPI) 17 as recommended by several studies:18 in Spain there was a 41.6% increase in prices between years 2000 and 2015, which yielded an average VSL result of 3,355,920€ in year 2015. The hemovigilance report outlines the age and gender of the transfused patient's deaths. Taking into account Spain's life expectancy of each gender,19 we calculated the value of a statistical year lived. The social cost of the transfused recipient's deaths was calculated considering the remaining years to achieve the average life expectancy. Recipient's deaths with an age over the average life expectancy were considered of 0 value in terms of social cost attributed to mortality. The social cost associated with mortality attributed to AEs was obtained as a sum of the different VSL of each death.
Methodology for the update of the transfusion unitary costIn order to update the total cost of a blood transfusion unit in Spain we have used the cost set at 350€ for the NHS in Spain as reported by Darbà et al.20 Such cost (set in Euros of the reference year 2007) refers to transfusions and therefore it is only the health care cost. Thus, to update the cost values to Euros of year 2015, we did not use the overall increased general CPI as in the case to update the VSL. Instead, we have used the corresponding CPI for healthcare activity according to the Classification of Individual Consumption by Purpose (COICOP) as recommended by Scharff.21 The cumulative increase in CPI for health care activity from year 2007 to 2015 has been 5.1%. Lastly, the addition of the AEs’ healthcare plus social costs is needed to assess the total cost.
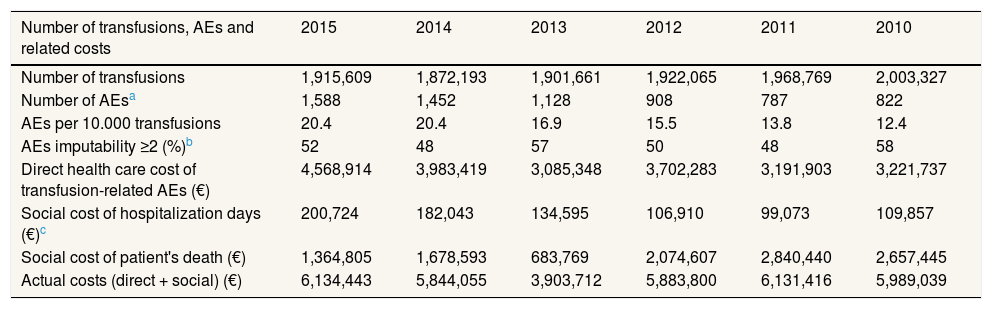
ResultsIn year 2015, in Spain, 1,915,609 transfusions were performed, concerning a population of 46,439,864 inhabitants. Of these, 1,588 patients showed transfusion-related AEs with an imputability level equal to or greater than 2. This figure has increased 93% since the last five years. The total cost of these AEs for the NHS was 6,134,443€ calculated as a sum of healthcare cost (4,568,914€), social cost as a result of lost working days due to hospitalization (200,724€) and social cost of patient's death (1,364,805€).
The trend in costs analyzed for the period 2010-2015 show an increase in the healthcare cost of transfusion-related AEs of 42% and in the social cost due to loss of productivity of 83%. The number of patient's deaths (between 4 and 2) and patient's age at the time of the death (between 33 and 90 years old) in the period analysed present a high variability and therefore its associated social costs (range: 2.84-0.68M€). As a sum 33.8M€ of extra expenses, due to transfusion related AEs, were calculated for the period 2010-2015 for the NHS. In terms of AEs reported per 10,000 transfusions, the ratio has increased from 12.4 in year 2010 to 20.4 in year 2015. Results are shown in Table 2.
Healthcare cost of transfusion-related adverse eventsThe total health care cost of the transfusion related AEs in Spain in year 2015 was 4,568,914€. The trend for the period 2010-2015 show an increase in the healthcare cost of transfusion-related AEs of 42%.
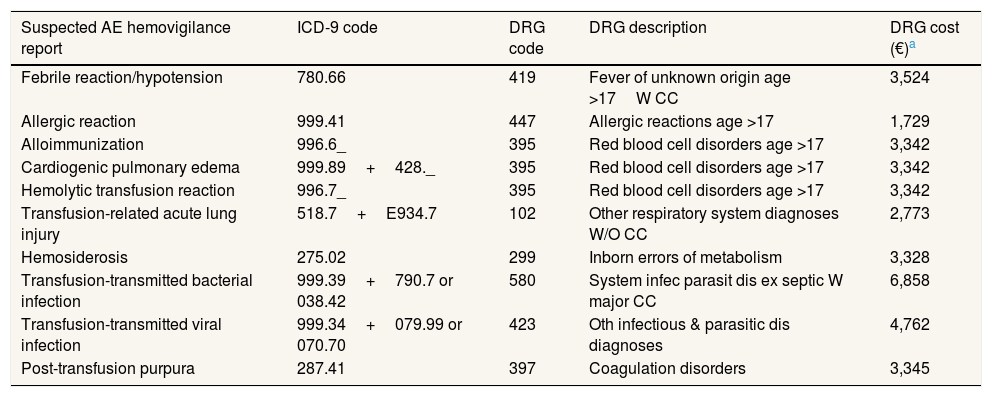
Following the methodology described before, the allocated unitary costs of transfusion-related AEs accounted in the hemovigilance report are shown in Table 3.
Allocation of transfusion-related adverse events to Diagnosis Related Groups and corresponding cost in year 2015 in Spain.
| Suspected AE hemovigilance report | ICD-9 code | DRG code | DRG description | DRG cost (€)a |
|---|---|---|---|---|
| Febrile reaction/hypotension | 780.66 | 419 | Fever of unknown origin age >17W CC | 3,524 |
| Allergic reaction | 999.41 | 447 | Allergic reactions age >17 | 1,729 |
| Alloimmunization | 996.6_ | 395 | Red blood cell disorders age >17 | 3,342 |
| Cardiogenic pulmonary edema | 999.89+428._ | 395 | Red blood cell disorders age >17 | 3,342 |
| Hemolytic transfusion reaction | 996.7_ | 395 | Red blood cell disorders age >17 | 3,342 |
| Transfusion-related acute lung injury | 518.7+E934.7 | 102 | Other respiratory system diagnoses W/O CC | 2,773 |
| Hemosiderosis | 275.02 | 299 | Inborn errors of metabolism | 3,328 |
| Transfusion-transmitted bacterial infection | 999.39+790.7 or 038.42 | 580 | System infec parasit dis ex septic W major CC | 6,858 |
| Transfusion-transmitted viral infection | 999.34+079.99 or 070.70 | 423 | Oth infectious & parasitic dis diagnoses | 4,762 |
| Post-transfusion purpura | 287.41 | 397 | Coagulation disorders | 3,345 |
AE: adverse event; DRG: Diagnosis Related Groups.
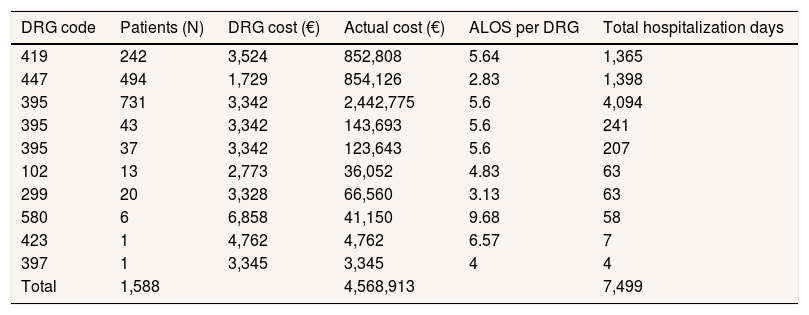
The health care cost of transfusion-related AEs was obtained using the methodology already described with data on numbers of AEs recorded in the hemovigilance report. Results are shown in Table 4.
Health care cost and hospitalization days due to transfusion-related adverse events in year 2015 in Spain.
| DRG code | Patients (N) | DRG cost (€) | Actual cost (€) | ALOS per DRG | Total hospitalization days |
|---|---|---|---|---|---|
| 419 | 242 | 3,524 | 852,808 | 5.64 | 1,365 |
| 447 | 494 | 1,729 | 854,126 | 2.83 | 1,398 |
| 395 | 731 | 3,342 | 2,442,775 | 5.6 | 4,094 |
| 395 | 43 | 3,342 | 143,693 | 5.6 | 241 |
| 395 | 37 | 3,342 | 123,643 | 5.6 | 207 |
| 102 | 13 | 2,773 | 36,052 | 4.83 | 63 |
| 299 | 20 | 3,328 | 66,560 | 3.13 | 63 |
| 580 | 6 | 6,858 | 41,150 | 9.68 | 58 |
| 423 | 1 | 4,762 | 4,762 | 6.57 | 7 |
| 397 | 1 | 3,345 | 3,345 | 4 | 4 |
| Total | 1,588 | 4,568,913 | 7,499 |
ALOS: average length of stay; DRG: Diagnosis Related Groups.
The social cost of lost working days in Spain was 200,724€. The trend for the period 2010-2015 show an increase of 82% in the social cost due to loss of productivity because of hospitalization days as a result of transfusion-related AEs.
The ALOS corresponding to year 2015 is shown in Table 4 in relation to DRG linked to transfusion-related AEs. A total of 7,499 hospitalization days, as a sum of all ALOS, was calculated for patients with transfusion-related AEs. Thus, the social cost of lost working days in year 2015 was calculated by multiplying the average daily salary computed previously (62.83€) by the sum of hospitalization days, and resulted in 471,183€. Taking into account that only 42.6% of all transfused patients fall into working-age population, the final social cost for loss of productivity due to AEs is 200,724€ in year 2015.
Social cost of mortalityIn 2015, three AEs resulted in patient's death. The social cost of the mortality associated to blood transfusions was 1,364,805€. Both the number of patient's deaths and patient's age at the time of the death for the period 2010-2015 present a high variability, without a marked trend, yearly and therefore its associated costs (range: 2.84-0.68M€).
Both the nationwide average income used to calculate the social cost of lost working days and the VSL used for calculating the social cost of mortality change annually. Table 2 shows the actual costs of transfusion-associated morbidity and mortality in recent years considering annual variations.
Principal ratios and actual costs of transfusion-related morbidity and mortality in years 2010-2015 in Spain (all figures are expressed in Euros 2015).
| Number of transfusions, AEs and related costs | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 |
|---|---|---|---|---|---|---|
| Number of transfusions | 1,915,609 | 1,872,193 | 1,901,661 | 1,922,065 | 1,968,769 | 2,003,327 |
| Number of AEsa | 1,588 | 1,452 | 1,128 | 908 | 787 | 822 |
| AEs per 10.000 transfusions | 20.4 | 20.4 | 16.9 | 15.5 | 13.8 | 12.4 |
| AEs imputability ≥2 (%)b | 52 | 48 | 57 | 50 | 48 | 58 |
| Direct health care cost of transfusion-related AEs (€) | 4,568,914 | 3,983,419 | 3,085,348 | 3,702,283 | 3,191,903 | 3,221,737 |
| Social cost of hospitalization days (€)c | 200,724 | 182,043 | 134,595 | 106,910 | 99,073 | 109,857 |
| Social cost of patient's death (€) | 1,364,805 | 1,678,593 | 683,769 | 2,074,607 | 2,840,440 | 2,657,445 |
| Actual costs (direct + social) (€) | 6,134,443 | 5,844,055 | 3,903,712 | 5,883,800 | 6,131,416 | 5,989,039 |
AEs: adverse events.
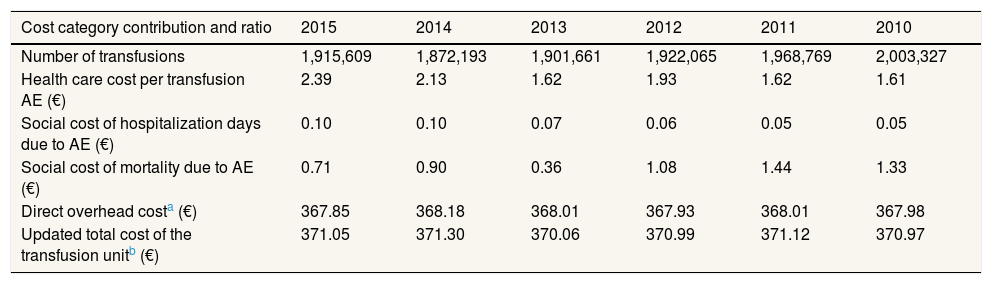
Following the methodology described before, the value of the blood transfusion unitary cost, including social costs, updated for year 2015 was 371.05€, calculated as follows. Firstly, the update to euros 2015 of the last transfusion unitary cost calculated in Spain in year 2007:20
350€ (€ 2007) × 5.1% (incremental years 2007-2015 CPI health care activity) =367.85€ (€ 2015)
Secondly, the addition of the direct healthcare and social cost category values attributed to the post-transfusion AEs: 2.39€ and 0.82€, respectively.
Taking into account the number of transfusions performed yearly in Spain, the update to euros 2015, plus the addition of the transfusion-related AEs’ costs, the trend in the updated cost of the transfusion unit in Spain is shown in Table 5.
Evolution of cost category contribution and ratio per blood transfusion unit in Spain (all figures are expressed in Euros 2015).
| Cost category contribution and ratio | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 |
|---|---|---|---|---|---|---|
| Number of transfusions | 1,915,609 | 1,872,193 | 1,901,661 | 1,922,065 | 1,968,769 | 2,003,327 |
| Health care cost per transfusion AE (€) | 2.39 | 2.13 | 1.62 | 1.93 | 1.62 | 1.61 |
| Social cost of hospitalization days due to AE (€) | 0.10 | 0.10 | 0.07 | 0.06 | 0.05 | 0.05 |
| Social cost of mortality due to AE (€) | 0.71 | 0.90 | 0.36 | 1.08 | 1.44 | 1.33 |
| Direct overhead costa (€) | 367.85 | 368.18 | 368.01 | 367.93 | 368.01 | 367.98 |
| Updated total cost of the transfusion unitb (€) | 371.05 | 371.30 | 370.06 | 370.99 | 371.12 | 370.97 |
AE: adverse event.
Our study calculates for the first time in Spain the total cost of transfusion-related AEs, including social costs. The number of transfusion related AEs show a 93% increase for the period 2010-2015 and their associated costs accounted for 6.13M€ in 2015. The study also describes a methodology for the calculation of the health care and social costs of morbidity and mortality associated to blood transfusion nationwide that can be easily conducted at the international level. All exploration objectives considered have been accomplished.
AEs in medicine represent a major problem for NHSs. Related to transfusions, both notifications of AEs and their associated costs, more specifically direct healthcare and social cost due to hospitalization, show a sharp upward trend during the last years. Post transfusion AEs in Spain accounted for 3.2€ per unitary packed red blood cell transfused, as a sum of healthcare costs and social costs due to such AEs. Cost-benefit studies should take into account all AEs’ related costs, including social ones, on behalf of accurate results and conclusions which could determine the adoption of cost-effectiveness new technologies and procedures.
In 2015, in the United Kingdom, over 2,577,276 transfusions, 3,965 AEs were reported, that is 0,154%.22 Compared to Spain, 3,043 AEs to transfusions (1,588 with imputability ≥2) were reported in the same year, representing a similar ratio of 0,159%. As mentioned in Table 2, in Spain, reported AEs ratio per 10,000 transfusions have grown from 12.4 in year 2010 to 20.4 in year 2015. In United Kingdom, in the same period, the ratio has increased from 11 to 15.4. Although showing similar prevalence between countries of the ratio of reported AEs, the difference may be due to the fact that the hemovigilance notification service in United Kingdom started in 1996, while in Spain the National Hemovigilance Unity was created in 2004 23 and serious AEs started to be notified in a regulated form in 2007.24
DRG and ICD-9 coding systems are in widespread use worldwide and the coding and clinical description of each category matches in every country.7,8 The present study describes a methodology that allows to know in a simple and explicit way the economic cost of transfusion-related AEs. DRG costs and their related ALOS vary each year. Every yearr's variations have been considered an accounted in the results.
It is of great importance to estimate not only the adeverse events¿ direct costs as recomended by Amin et al.25 and Agrawal et al.26 but also the social costs of blood transfusion-related AEs for an adequate economic assessment, as recommended by Neumann et al.27 In the present study, social costs, including loss of productivity and mortality associated to post-transfusion AEs, accounted for 4.4% and 29% respectively of all AEs’ costs.
In a similar way as the imputability of the AE associated to transfusions showed in Table 1, the hemovigilance report scores the severity of every AE. However, such severity scoring has not been considered for the calculation of the health costs associated to transfusion-related AEs, because the cost of each DRG is already a weighted average of several clinical episodes with different severity grouped by a similar clinical presentation.
The trend in the number of blood units transfused is decreasing during the last few years in Spain, as shown in Table 2, in part due to implementation of blood-saving policies,28 to the awareness of health care workers, the adaptation of surgical practices to less invasive procedures,29 and to the development of novel drugs and protocols.30 Demographically, Spanish population has descent 0.1% between years 2010 and 2015, while the population aging has increased. From 16.7% of Spanish population over 65 years of age in year 2010 to 18.1% in year 2015.31
However, it must be noted the steep increase in the actual healthcare cost associated to transfusion-related AEs in recent years. This increase is not due to a greater number of transfusions performed, but rather to a greater number of reported AEs, from 0.079% per blood unit transfused in year 2010 to 0.159% in year 2015, that is, increased by 100%. The likely reason to explain the increase in the number of AEs recorded is that it is becoming more common to notify them in recent years, rather than a true greater occurrence of post-transfusion-related AEs with respect to previous years. This notion is reinforced with progressive adherence of hospitals from the NHS to centralized information system of the Ministry of Health, together with a greater awareness of the importance of such notifications. Of course, the first step to be aware of its importance is to know the actual costs. In clinical practice, AEs are a serious problem with very important implications 32 that may represent more than 15% of the overall expenditure of a hospital.33
A limitation of this study is that it must be noted that not all social costs of the blood transfusion process have been fully considered. Besides the social costs computed in the present study, other minor blood donation-associated costs might be added. For example, the social donor's opportunity cost in terms of lost working days as reported by Amin et al.,25 the indirect cost associated with recruiting and retaining blood donors, as well as the healthcare cost of AEs associated to blood donation (bruising, urticaria, itching, nerve injury, vasovagal reactions, thrombophlebitis, etc.). Such complications are usually less frequent and milder than those assessed in the present study, and they likely contribute a much lower cost compared with the AEs recorded; although, they represent a true cost for the NHS. Moreover, not all patients get to work immediately when receiving hospital discharge. The average salary used in the calculation of the social cost due to ALOS does not include the social security contribution made by firms or autonomous workers, since there is no data available of a national average of social security payments that both enterprises and autonomous workers spend in social security coverage. Therefore, it is probable to have underestimated slightly the actual cost of the transfusion.
Post-transfusion morbidity and mortality costs account for 6.13M€ yearly in Spain and not only the healthcare cost, but also the number of AEs notified show an upward trend during recent years of 42% and 93% respectively. These costs and tendencies may alert health care providers and should be taken into account to update cost-effectiveness trials and for future studies regarding design and assessment of processes or techniques involving blood saving or transfusion activities. Finally, because there is a current trend for economic evaluations to consider the social costs and even patients’ health preferences,34 it is therefore concluded that the analysis of social costs described in this study may be of great use when making decisions in the field of public health.
NHS’ budget faces increasing pressure yearly. Sanitary decision makers need to deal constantly between adoption of innovative health technology, and the restricted economic resources that every Ministry manages. Future studies regarding AEs with a continuous update in costs are necessary to help NHS decision policies and a more rapidly adoption of cost-effectiveness technology.
In Spain, the cost of a packed red blood cell in year 2007 was estimated to be 150€ per unit and 350€ per transfusion process. However, adverse events and social costs related to transfusions should also be considered to estimate a more precise cost of transfusions.
What does this study add to the literature?The study analyses for the first time in Spain the total (including social) costs of transfusion-related adverse events and explains a methodology that can be conducted globally to obtain precise transfusion costs information. Costs analyzed should be taken into account to update cost-effectiveness trials regarding blood or transfusion activities, while the tendencies analysed should alert health care providers when making decisions in public health.
David Epstein.
Transparency declarationThe corresponding author on behalf of the other authors guarantee the accuracy, transparency and honesty of the data and information contained in the study, that no relevant information has been omitted and that all discrepancies between authors have been adequately resolved and described.
Authorship contributionsAll authors participated in the study design and revised and improved successive versions of the manuscript. B. Ribed Sánchez: first author. C.González Gaya: global reviewer, cost modelization and update. S. Varea Diaz: hemovigilance reports check, update and global review. C. Corbacho-Fabregat: global reviewer and technical consultant. I. Bule Farto: DRG, CIE-9 and CIE-10 expert. Contribution in cost assignment and review. J. Pérez de Oteyza: global transfusion adverse reaction check and review. All authors have approved the manuscript final version.
FundingNone.
Conflicts of interestNone.
Cristobal Belda Iniesta and Angel Ayuso Sacido critically revised the manuscript.