The TackSHS project aims to comprehensively elucidate the impact that exposure to second-hand smoke (SHS) from cigarettes and second-hand aerosols (SHA) from electronic cigarettes have on the respiratory health of the European population according to socioeconomic characteristics and other determinants.
MethodThe TackSHS project involves a series of coordinated studies carried out by 11 academic and public health organisations from six European countries. The project will investigate: a) the determinants of SHS and SHA exposure assessed at the individual level (surveys on representative general population samples) and in common environments (environmental sampling in specific settings); b) the overall disease burden, mortality and morbidity attributable to such exposure; and c) its economic impact in terms of direct health care costs. The project will also examine specific acute respiratory health changes in healthy individuals and patients with respiratory diseases exposed to SHS and SHA. In addition, the project will examine the effectiveness of a novel intervention to reduce SHS exposure in households where smoking is permitted. All these studies are inter-related and involve collaborative coordination among the participant organisations.
ConclusionThe comprehensive, integrated approach of the TackSHS project will enable a significant step forward from the current status quo in the understanding of the impact of SHS and SHA exposure on health and provide the basis for health policy recommendations to help European countries to further reduce the harm caused by SHS and SHA exposure.
El proyecto TackSHS pretende caracterizar el impacto global de la exposición al humo ambiental de tabaco (HAT) y al aerosol de los cigarrillos electrónicos (ACE) en la salud respiratoria de la población europea según variables socioeconómicas y otros determinantes.
MétodoEl proyecto TackSHS consiste en una serie de estudios coordinados y gestionados por 11 organizaciones académicas y de salud pública de seis países europeos. El proyecto estudiará: a) los determinantes de la exposición al HAT y al ACE a nivel individual (encuestas en muestras representativas de la población general) y en espacios comunes (muestras ambientales en lugares específicos); b) su carga general de enfermedad y la morbimortalidad atribuible a tal exposición; y c) su impacto económico en términos de costes sanitarios directos e indirectos. Además, el proyecto investigará cambios específicos a corto plazo en la salud respiratoria en personas sanas y en pacientes con enfermedades respiratorias expuestos al HAT y al ACE. También examinará la efectividad de una intervención novedosa para reducir la exposición al HAT en hogares donde se permite fumar. Todos estos estudios están interrelacionados y conllevan una coordinación colaborativa entre las instituciones participantes.
ConclusiónEl enfoque integral del proyecto TackSHS permitirá un avance significativo en la evidencia sobre la comprensión del impacto de la exposición al HAT y al ACE en la salud, y proporcionará una base para desarrollar recomendaciones políticas sanitarias para ayudar a los países europeos a reducir los daños causados por la exposición al HAT y al ACE.
Research produced during the past three decades has provided substantial evidence of the harm that short- and long-term exposure to second-hand smoke (SHS) represents to respiratory and cardiovascular health of adults and children.1,2 While interest in SHS exposure prevalence and its effects is increasing,3 important gaps remain when designing national and international health policies aiming to protect populations. One of them is a scarcity of studies objectively measuring markers of SHS in settings where smoking is not regulated, such as private and outdoor public places.
In addition, electronic cigarettes (e-cigarettes) have irrupted into the market of tobacco products in the past decade, creating an on-going debate among the research community on their health effects and potential impact on public health.4 While active e-cigarette use and its health effects have received substantial attention in research, evidence on the health impact of bystanders’ exposure to second-hand aerosols (SHA) generated by its use is still scarce.5
The project “Tackling secondhand tobacco smoke and e-cigarette emissions: exposure assessment, novel interventions, impact on lung diseases and economic burden in diverse European populations” (the TackSHS project: www.tackshs.eu) was developed to address these and other challenges in the research of SHS and SHA. The TackSHS project intends to comprehensively elucidate the impact that SHS and SHA exposure have on the European population and how health impacts vary according to socioeconomic and other demographic characteristics, with a particular emphasis on specific vulnerable groups. This paper describes the specific objectives and methods of each study or ‘work-package’ within the project.
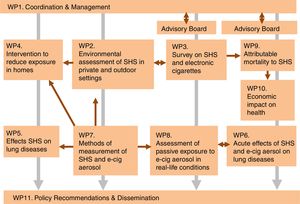
MethodThe TackSHS project is organised in 11 work-packages (WP) of which eight are research-oriented and correspond to independent studies, with a further three WPs involved in providing support (coordination, instrument calibration, and results dissemination) during the 48 months of the project (November 2015 – October 2019). The overall structure of the project is depicted in Figure 1. A summary of the WPs with the leading partners is provided in Table I of the online Appendix.
This is a European cross-sectional study, led by the Agència de Salut Pública de Barcelona (Spain), that aims to objectively assess SHS exposure across a range of different environments where smoking was not regulated at the time this project was planned. The study will be conducted in 11 European countries (Bulgaria, France, Germany, Greece, Ireland, Italy, Poland, Portugal, Romania, Spain and the United Kingdom) representing geographical, legislative (country-specific smoke-free policies) and cultural variations across the European Union (EU). Private settings studied will include homes and cars, while outdoor settings will include terraces of hospitality venues, children playgrounds and entrances to primary school buildings. Around 1000 environmental measurements will be collected: 180 measurements from homes in 9 countries and 660 measurements from outdoor settings in the 11 countries involved in the study; and 120 environmental measurements will be collected in cars in two countries (Spain and the United Kingdom). These countries were selected considering differences in smoking legislation in cars. A convenience sample will be obtained considering neighbourhoods from different socioeconomic status, which will be assessed using local ecological synthetic indexes. SHS will be assessed through the measurement of airborne nicotine. Nicotine samplers, which consist of a 37-mm diameter plastic cassette containing a filter treated with sodium bisulphate, will be used. A passive sampling will be performed in homes (7-day sampling) and cars (24-hour sampling). In outdoor settings, active sampling will be used attaching a nicotine sampler to an air pump with a flow rate of 3 L/min, and 30-min measurements will be taken. Sampling will be done avoiding the warmest and the coldest months of the year. All the nicotine samples will be analysed by gas chromatography/mass spectrometry.6
Study 2. The TackSHS survey: a pan-European study on SHS and SHA exposure (WP3)This is a European cross-sectional study, led by the Istituto di Ricerche Farmacologiche Mario Negri IRCCS (Italy), that aims to: 1) estimate the prevalence and the determinants of smoking, e-cigarette use, and exposure to SHS and SHA; 2) analyse attitudes, perceptions and behaviours of the adult European population towards smoke-free policies in their countries; and 3) compare selected smoking-related data originated in this study and a previously conducted pan-European survey (i.e., the PPACTE project).7 Approximately 12,000 individuals aged 15 years and older will be enrolled in 12 EU countries (the same countries listed for WP2 plus Latvia). The TackSHS survey will be representative of the country-specific population using a similar methodology of a previous project.7 The sample size in each specific country (approximately n=1000) will enable the estimation of prevalence in that country with a standard error of±1.6%. The sampling methodology (in most of the countries multistage/stratified random sampling) will ensure the representativeness of the adult population in terms of sex, age, geographic area and selected socioeconomic characteristics. A standard questionnaire developed ad hoc from existing validated questionnaires will be used to collect information on cigarette smoking, use of e-cigarettes and emergent heated tobacco products. The questionnaire focuses particularly on the exposure to SHS and the passive exposure to SHA from e-cigarettes in different indoor and outdoor settings. WP3 will also examine compliance with the current country-specific smoke-free legislations, attitudes and perceptions towards various tobacco control policies, including smoking ban regulations that are already adopted, and an extension of these regulations to other (outdoor) public places. Finally, the survey will explore participants’ knowledge of the harmful effects of SHS and SHA.
Study 3. Air quality feedback to reduce SHS exposure in homes (WP4)This is an intervention study, led by the University of Stirling (Scotland, UK), aiming to encourage behaviour-change towards a smoke-free home environment. The study will provide the participants (smokers who smoke at home and take care of at least one child) regular feedback in the form of personalised air quality measurements made in their homes. This study will develop a targeted intervention with socioeconomically deprived smokers in four countries (Greece, Italy, Scotland, and Spain) based on a previously piloted intervention.8 The study will aim to recruit 160 smokers (40 in each country). Participants will have particle counting instruments to measure in real time particulate matter concentration (PM2.5) installed in their homes for a period of 30 days. These devices will allow data acquisition in real-time with a 3G internet connection. During this period, personalised feedback will be provided to, and discussed with, the smoker along with target-setting and exploration of suitable methods of behaviour-change. Personalised feedback will be given via text messages to mobile phones, email messages and through telephone calls. A final home visit will gather data on changes made while some participants (10-20%) will take part in a further qualitative phone interview to gather data on their experience of the intervention.
Study 4. Health effects of SHS exposure in outdoor smoking areas in patients with COPD and asthma (WP5)This is a clinical research study, led by TobaccoFree Research Institute Ireland, aiming to assess the acute respiratory health effects that result from short-term SHS exposure among patients with asthma and chronic obstructive pulmonary disease (COPD). In many European countries, the introduction of smoke-free laws has prompted the proliferation of outdoor areas where smoking is permitted, and there exist places where non-smokers are still exposed to some levels of SHS. The health effects of such exposures are uncertain as to date there is only anecdotal evidence; some effects can be expected but it may be difficult to demonstrate unless in naturalistic, real-life conditions. This study will aim to involve 60 patients (volunteers) from three European countries: the Czech Republic, Ireland, and Spain (20 patients in each participating country: 10 COPD and 10 asthma patients). The recruitment of such patients will be done with the cooperation of patients’ organisations in these countries which are affiliated with the European Lung Foundation. Participants will be trained to keep a diary record of any noted changes and medication usage and perform pre- and post-peak flow, spirometry, and carbon monoxide measurements. Such patients will wear personal monitors to continuously measure exposure to particulate matter (PM1, PM2.5, PM10) with continuous geolocalisation monitoring with AirSpeck mobile wireless air quality monitors and a RESpeck monitor to measure breathing rate and depth to detect any acute changes in breathing during or following exposure to SHS at entertainment facilities. These novel measurements will determine the individual level of SHS exposure with spatially-resolved estimation of personal dosage of PM and the identification of acute respiratory effects from such exposure. There is also a recording of each breath sound which can be recognised and matched with time, location and level of exposure with identification of any lags in time that may occur.
Study 5. Impact of short-term second-hand exposure to e-cigarette aerosols (SHA) on the respiratory system (WP6)This study, led by the Hellenic Cancer Society (Greece), is aimed at characterizing exposure to e-cigarette aerosols among healthy non-smoker volunteers. An experimental study with cross-over design will be implemented in a laboratory. Three trial arms will be performed (no exposure vs. low e-cigarette aerosol exposure vs. high e-cigarette aerosol exposure) that will be differentiated by the e-cigarettes’ battery power outputs. The study sample will consist of 40 adult volunteers that will be randomized to the sequence of three exposures to e-cigarette aerosol. In total, 120 person-exposures will take place, leading to 240 pre- and post- measurements. Recruitment will take place in Athens (Greece). Exposures will last 30minutes and will take place in a standardised exposure chamber.9 Before and after exposure to aerosols originated from e-cigarette use, the lung function of each participant will be assessed through impulse oscillometry to assess respiratory system impedance and resistance, exhaled nitric oxide levels and 8-isoprostane concentration in the exhaled breath condensate as a marker of oxidative stress.10
Study 6. Second-hand exposure to emissions from electronic cigarettes: personal and environmental assessment in confined spaces (WP8)This WP, led by the Institut Català d’Oncologia (Spain), comprises three sub-studies to address three complementary objectives. Firstly, a systematic review of the published studies on passive exposure to e-cigarette aerosols will be performed. Secondly, environmental and bystanders’ exposure to e-cigarette aerosols in controlled conditions in a car and a room will be investigated through an experimental study which will involve two volunteers exposed to SHA and an e-cigarette user to produce it. Airborne nicotine and PM2.5 will be measured for environmental exposure and nicotine, cotinine and tobacco-specific nitrosamines in saliva for personal exposure assessment. Thirdly, a cross-sectional study will be conducted to investigate environmental and bystanders’ exposure to SHA in real-life conditions at homes of e-cigarette users, in four European countries (Greece, Italy, Spain, and the UK). Based on the differences detected in a previous pilot study11 and standard assumptions, this study will aim to include 250 participants. Airborne nicotine and PM2.5 will be measured during one week and saliva and urine samples will be collected for the determination of nicotine, cotinine and tobacco-specific nitrosamines. Airborne nicotine samples will be analysed by gas chromatography/mass spectrometry;6,12 while saliva and urine analysis will be performed using high-resolution chromatography/mass spectrometry;13,14 and PM2.5 will be measured by means of an optical portable counter.15
Study 8. Attributable mortality and morbidity to SHS in Europe (WP9)The objectives of this WP, led by the Istituto per lo Studio, la Prevenzione e la Rete Oncologica (Italy), are: 1) to review the methods used in the literature, with special focus on Europe, to calculate attributable mortality and morbidity to SHS and the results published to date for EU countries; 2) to develop algorithms of calculation of attributable mortality and morbidity to SHS; and 3) to update attributable mortality and morbidity to SHS for EU countries based on SHS exposure prevalence collected in the WP3.
In order to estimate attributable mortality and morbidity to SHS, mortality and morbidity data (asthma, COPD, lung cancer, stroke, ischaemic heart disease, breast cancer and diabetes in adults; low birth weight, lower respiratory infections, otitis media, asthma and sudden infant death syndrome in children) will be collected from 28 EU countries on SHS-related diseases, along with information on prevalence of SHS exposure in EU countries. For attributable mortality and morbidity estimates, data on the prevalence of SHS exposure will be used from two sources: information collected in the WP3 of this project and from Eurobarometer surveys. Finally, sensitivity analyses of attributable mortality and morbidity to SHS will be conducted.
Study 9: Economic impact of SHS on morbidity and mortality and return on investment of interventions aiming at reducing SHS exposure (WP10)This study, led by the Universidad Politécnica de Cartagena (Spain), will produce economic models for the analysis of the return on investment of policies aimed at reducing SHS exposure in EU countries. Available return on investment models for smoking cessation interventions (http://www.equipt.eu) will be considered. Nevertheless, these models consider the effects of SHS exposure by assuming that each successful quit contributes a fractional reduction in the disease burden associated with SHS, they do not include policies specifically aimed at reducing SHS other than curbing smoking prevalence. The information on SHS exposure in European countries obtained in the WP3 and the epidemiological evidence on attributable mortality and morbidity to SHS obtained in the WP9 will be combined with data on the cost consequences of disease to obtain country-specific versions of the return on investment models. The models will consider the lag between exposure and effect and therefore will use appropriate short- and medium-term horizons (2, 5, and 10 years) and long-term horizons (20 years and lifetime). With these models, the economic burden caused by SHS exposure and the return on investment of some interventions will be estimated. Different perspectives (health system, overall public sector and wider society) will be adopted. The model outcomes will comprise not only standard cost-effectiveness and cost-utility ratios but also a fully disaggregated list of health outcomes and resource use consequences so that the budgetary impact of implementation at local, regional and national scale will be appraised.
Supportive WP: Instruments’ calibration (WP7)This WP, led by the Fondazione IRCCS Istituto Nazionale dei Tumori (Italy), will investigate the best methods and procedures for real-time measurement of SHS and SHA using optical particle counters. All devices of the project Partners used in WP4, WP5, WP6 and WP8 (Dylos DC1700, SidePak AM150, and AirVisual Pro) will be tested and individually calibrated through comparison with one Beta Attenuation Monitor (BAM-1020).
The Dylos DC1700 is an optical particle counter instrument that counts the small and large particles present in the surrounding environment. The TSI SidePak AM510 Personal Aerosol Monitor is a laser photometer that measures a variety of particle sizes (PM1, PM2.5, PM10). The AirVisual Pro is a low-cost optical particle counter, measuring PM2.5, PM10, carbon dioxide, temperature and relative humidity. All these devices are portable and stationary, capable to operate and memorise the data with or without connection to the internet.
For calibration, comparison of different measurements will be performed by the generation of exposures using a range of cigarettes and e-cigarettes in a real-life environment in a room of 48 m3. The BAM-1020 will be operated and the optical particle counters will be calibrated in parallel for the time necessary to simulate concentration changes in order to verify the accuracy and precision of the instruments within the whole range of measurements specified by the manufacturers and to compensate for coincidence losses.
ConclusionThe TackSHS project involves a comprehensive and innovative approach to develop a scientific understanding of second-hand exposure to smoke from cigarettes and aerosols from e-cigarettes. For the first time, this project will consider the integration of data on SHS exposure from a validated, homogenous survey, as an instrument for the subjectively perceived exposure by wide populations in several EU countries, together with its objective assessment using environmental and biological markers. Whilst this is the core objective of the TackSHS project, additional WPs will address specific questions regarding exposure to SHS and SHA, including its impact on health and its economic burden in specific populations and patients with chronic lung and respiratory diseases.
At the societal level, we aim to raise awareness of the risks from SHS exposure and their associated health burden. The project will also provide new evidence to further inform the debate about the scale of risk from SHA exposure. TackSHS team will also invest considerable effort in disseminating the obtained results, not only to the scientific community, policy makers and stakeholders, but also to patient and consumer organisations at the national and European level.
The initial publication plan includes 20 papers with the main findings, considered as priority publications. Further publications, with results at the country level and combining the results from different studies will be produced after the initial primary publications. In addition, a comprehensive report, as well as policy briefs, will be launched in the final conference and distributed to stakeholders, including policymakers, patients, the scientific community and the general public.
Editor in chargeCarlos Álvarez-Dardet.
Authorship contributionsE. Fernández and M.J. López designed the overall framework of the project protocol. E. Fernández, M.J. López, S. Gallus, S. Semple, L. Clancy, P. Behrakis, A. Ruprecht, G. Gorini, C. Radu-Loghin, J.B. Soriano and A. López-Nicolás developed detailed protocols of the corresponding studies they coordinate. All the TackSHS project investigators have participated in different phases of the project, contributed to this manuscript and gave final approval. E. Fernández prepared a first complete draft of this manuscript; all authors critically reviewed it and contributed with significant and important suggestions. All authors have participated in writing the manuscript, its critical review and have approved the final version. All of them are jointly responsible for adequate revision and discussion of all aspects included in the manuscript.
AcknowledgementsWe thank Ms. Cristina Rajo, formerly at the Research Support Office of the Catalan Institute of Oncology, for her valuable help throughout the process for the grant application and negotiation. We also thank Ana Navas-Acién, Dan Smith, Armando Peruga and James Repace for accepting being members of the Scientific Advisory Board, and Fèlix Bosch for accepting being the External Ethics Advisor of the project.
In memory of Dr. Manel Nebot (1957-2012) and Dr. Giovanni Invernizzi (1948-2013), whose work and friendship guided us in this and other projects.
FundingThis project has received funding from the European Union Horizon 2020 research and innovation programme under grant agreement No 681040. The Tobacco Control Unit (ICO) is partially supported by the Ministry of Universities and Research, Government of Catalonia (2017SGR139). E. Fernández was partially supported by the Instituto de Salud Carlos III, Government of Spain (INT16/00211 and INT17/00103), co-funded by the European Regional Development Fund (FEDER). A. Lugo was supported by an AIRC fellowship for Italy. The work of S. Gallus was partially funded by the Italian League Against Cancer (LILT, Milan).
Conflicts of interestsThe authors declare no conflicts of interests. Specifically, they declare not receiving, directly or indirectly, funding from tobacco manufacturers or their affiliates.
Catalan Institute of Oncology (ICO), Bellvitge Biomedical Research Institute (IDIBELL), Spain: Esteve Fernández, Yolanda Castellano, Marcela Fu, Montse Ballbè, Beladenta Amalia, Olena Tigova; Public Health Agency of Barcelona (ASPB), Spain: María José López, Xavier Continente, Teresa Arechávala, Elisabet Henderson; Istituto di Ricerche Farmacologiche Mario Negri IRCCS (IRFMN), Italy: Silvano Gallus, Alessandra Lugo, Xiaoqiu Liu, Cristina Bosetti, Enrico Davoli; Istituto DOXA, Worldwide Independent Network/Gallup International Association, Italy: Paolo Colombo; University of Stirling (UNISTIR), UK: Sean Semple, Rachel O’Donnell, Ruaraidh Dobson; TobaccoFree Research Institute Ireland (TFRI), Ireland: Luke Clancy, Sheila Keogan, Shashsa Li, Elizabeth Breslin, Hannah Byrne; Hellenic Cancer Society - George D. Behrakis Research Lab (HCS), Greece: Panagiotis Behrakis, Anna Tzortzi, Constantine Vardavas, Vergina Konstantina Vyzikidou, Stephanie Teloniatis, Gerasimos Bakelas, George Mattiampa; Fondazione IRCCS Istituto Nazionale dei Tumori (INT), Italy: Roberto Boffi, Ario Ruprecht, Cinzia De Marco, Alessandro Borgini, Chiara Veronese, Martina Bertoldi, Andrea Tittarelli; Istituto per lo Studio, la Prevenzione, e la Rete Oncologica (ISPRO), Italy: Giuseppe Gorini, Giulia Carreras, Barbara Cortini, Simona Verdi, Alessio Lachi, Elisabetta Chellini; Polytechnic University of Cartagena (UPCT), Spain: Ángel López Nicolás, Marta Trapero-Bertran, Daniel Celdrán Guerrero; European Network on Smoking and Tobacco Prevention (ENSP), Belgium: Cornel Radu-Loghin, Dominick Nguyen, Polina Starchenko; Fundación para la Investigación Biomédica del Hospital Universitario La Princesa (IISP), Spain: Joan B. Soriano, Julio Ancochea, Tamara Alonso, María Teresa Pastor, Marta Erro, Ana Roca, Patricia Pérez.