We aimed to show an area-level association between the frequency of intestinal metaplasia (IM) in Helicobacter pylori-infected patients and tobacco consumption.
MethodsWe systematically reviewed the literature to retrieve data on the prevalence of IM in different countries and performed an ecological analysis to quantify the association between the prevalence of IM among infected subjects and smoking, using data on national tobacco availability. Articles evaluating IM in the general population or in dyspeptic patients were identified by a MEDLINE search. We selected one study per country, giving preference to those for which the study design/populations evaluated provided the highest external validity and inter-study comparability of methodology.
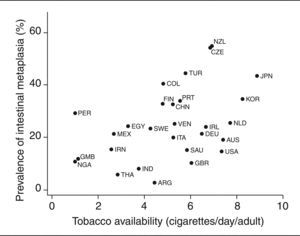
ResultsThis systematic review of published data retrieved information for 29 countries from 5 continents depicting a wide variation in the prevalence of IM among H. pylori-infected subjects in different regions, ranging from 3% in Argentina to 55% in New Zealand. In countries exhibiting a simultaneously high prevalence of infection and low incidence of gastric cancer, IM was also relatively infrequent (Thailand, 6%; India, 8.2%; Nigeria, 11.1%; Gambia, 11.8%; Saudi Arabia, 15.5%; Iran, 15.6%; Egypt, 24.4%). A significant correlation was observed between IM prevalence in infected subjects and tobacco availability (r = 0.45; p = 0.02).
ConclusionsOur results show that the concept of the African and Asian «enigmas» may be extended to precancerous lesions. Tobacco availability was positively associated with the prevalence of IM among H. pylori-infected subjects at an area level.
Evaluar la asociación entre la frecuencia de metaplasia intestinal (MI) en los sujetos infectados por Helicobacter pylori y el consumo de tabaco.
MétodosRevisión sistemática de la literatura médica para obtener datos de la prevalencia de MI en diferentes países y análisis ecológico para cuantificar la asociación entre prevalencia de MI en los infectados y la disponibilidad de tabaco en cada país. En una búsqueda a través de MEDLINE se identificaron artículos en los que se evalúan casos de MI en la población general o en pacientes dispépticos. Para seleccionar un artículo por cada país, se han elegido aquellos en que el estudio del diseño/población evaluado proporcionaba una mayor validez externa y comparabilidad.
ResultadosSe han seleccionado 22 artículos que contenían datos para 29 países de los 5 continentes, describiendo la gran variabilidad de la prevalencia de MI en los sujetos infectados en los diferentes países, desde el 3% en Argentina hasta el 55% en Nueva Zelanda. En los países que presentaban simultáneamente una prevalencia de infección alta y una incidencia de cáncer gástrico baja, la MI era también relativamente infrecuente (Tailandia, 6%; India, 8,2%; Nigeria, 11,1%; Gambia, 11,8%; Arabia Saudí, 15,5%; Irán, 15,6%; Egipto, 24,4%). Se observó una correlación significativa entre la prevalencia de MI en los infectados y la disponibilidad de tabaco (r = 0,45; p = 0,02).
ConclusionesNuestros resultados muestran que el concepto de «enigmas» africano y asiático se puede extender a las lesions precancerosas. Desde la perspectiva de un estudio ecológico, la disponibilidad de tabaco se asocia con la prevalencia de MI en los sujetos infectados por H. pylori.
Stomach cancer is one of the most frequent cancers, both in developed and developing countries, with a wide variation in incidence and mortality rates across geographical areas1. «Intestinal» gastric carcinomas, which are the most frequent variety and to which regional differences in the incidence of gastric cancer are attributed2, are preceded by atrophic gastritis, intestinal metaplasia (IM), and dysplasia. They follow a set of sequential steps3 which is amenable to modulation by environmental and individual susceptibility factors.
Helicobacter pylori is the most important4 risk factor for gastric cancer5,6 and its precursor lesions7,8. Among other environmental exposures, low fruit and vegetable consumption9 and smoking10,11 are established risk factors for gastric cancer. Several studies have also addressed the association between different lifestyles and the occurrence of precancerous gastric lesions, especially IM. Smoking is the most extensively studied of these lifestyle exposures and the one that most strongly increases the risk of IM and IM progression12-18. With respect to host genetic background, polymorphisms in genes coding for pro-inflammatory cytokines (interleukin-1B and its receptor antagonist) are responsible for increased susceptibility to the development of cancer19-21 and precancerous lesions22-24.
Regional differences in the virulence of H. pylori strains25,26, host genetic profile27,28 and response to infection29,30, and exposure to environmental hazards and certain lifestyles31-33 have all been proposed as explanations for the low incidence of gastric cancer in many countries with a high-prevalence of infection: the so-called African34 and Asian35 «enigmas».
Ecological32 and individual level36,37 evidence supports the hypothesis of a synergistic effect between H. pylori and smoking, which suggests that the low cigarette consumption found in most African and South-Eastern Asian countries contributes to the low frequency of gastric cancer in these regions, despite the high prevalence of infection. If an area-level association between the prevalence of IM amongst the infected and smoking could also be demonstrated, this would provide further support for the hypothesis that geographical variations in the consumption of tobacco could help to explain these «enigmas».
We thereby systematically reviewed the literature to retrieve data on the prevalence of IM in different countries and performed an ecological analysis aimed at quantifying the association between the prevalence of IM in H. pylori-infected subjects and tobacco availability in each country.
MethodsData on the prevalence of IM were obtained through a MEDLINE search using the following expression: metaplasia AND (stomach OR gastric) AND (prevalence OR incidence OR epidemiology OR epidemio* OR prev* OR incid*) NOT (case reports [ptyp])) AND (English[lang] OR French[lang] OR Italian[lang] OR Spanish[lang] OR Portuguese[lang]) AND (adult[MeSH]) AND (Humans[Mesh]) AND («1996/01/01»[EDat]:«2006/06/01» [EDat]) in Pubmed (http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?DB=pubmed). Four hundred and seventeen articles written in English, French, Italian, Spanish or Portuguese and published between January 1st, 1996 and June 1st, 2006 were retrieved.
One reviewer (BP) screened all the references and, after reviewing the titles and abstracts and/or the full articles, excluded those that were clearly unrelated to the study subject (e.g. laboratory research, family studies) or that had an aim or sampling strategy that resulted in the oversampling of subjects presenting atrophic gastritis, IM, dysplasia, adenocarcinoma, esophageal, duodenal (e.g. trials performed on subjects with gastric precancerous lesions, studies in which the sampling strategy did not include a random or consecutive sample of subjects undergoing upper digestive endoscopy) or other non-gastric conditions (e.g. cohorts or caseseries of patients with other diseases). When more than one figure was available for a given country, preference was given to studies that analyzed more than one country (to ensure greater comparability of the methods used to assess IM), had national coverage, evaluated random samples of the general population or had the largest sample size. The previous criteria were considered in the above-mentioned order in order to select one report per country. A flow-chart of the review is presented in figure 1.
Results from 29 countries, obtained from 22 articles, were included in the systematic review12,38-58. For each study, we extracted information on the country, year of publication (the dates when the studies where performed were only available for 5 studies), characteristics of the subjects evaluated, sample size, gender and age of the participants, biopsy sites, and IM prevalence in H. pylori-infected individuals, in non-infected subjects and in the sample as a whole. A summary of the characteristics of the studies included in the systematic review is presented in table 1 and data about IM prevalence is in table 2.
Characteristics of the studies included in the systematic review
| Country, reference | Characteristics of the subjects evaluated | Sample size (M/F) | Age (years)a | Biopsy sites (number of specimens) | Criteria for histological evaluation |
| Africa | |||||
| Egypt43 | Patients with dyspepsia | 92 (69/23) | 25-68 | Antrum (2) | Whitehead classification |
| Gambia44 | Adults with dyspepsia | 45 | 18-70 | Body (1) | Modified Sydney system |
| Antrum (2) | |||||
| Incisura (1) | |||||
| Nigeria51 | Review of endoscopic gastric biopsies with a histological diagnosis of chronic gastritis | 85 | Not stated | Not specified | Not specified |
| America | |||||
| Argentina38 | Symptomatic individuals who underwent upper gastrointestinal endoscopy as a control group for celiac disease cases | 114 (80/34) | 15-76 | Antrum (1) | Sydney system |
| Body (1) | |||||
| Colombia41 | Patients with nonulcer dyspepsia as a control group for gastric cancer cases | 67 (39/28) | 19-76 | Antrum (5) | Updated Sydney system |
| Corpus (6) | |||||
| Cardia (1) | |||||
| Mexico49 | Asymptomatic volunteers recruited through radio announcements for gastric cancer screening | 368 | > 40c | Antrum (3) | Updated Sydney system |
| Incisura (1) | |||||
| Corpus (3) | |||||
| Peru52 | H. pylori-infected participants from 2 different gastric cancer prevention programs, in which symptomatic patients were included | 78 (29/49) | 18-65 | Antrum (6) | Not specified |
| Body (4) | |||||
| Cardia (3) | |||||
| United States of America53 | H. pylori-infected individuals who underwent gastroscopic examination for mild epigastric symptoms or routine screening | 147 (71/76) | 57.4 ± 17.9b | Antrum (not specified) | Updated Sydney system |
| Body (not specified) | |||||
| Venezuela12 | Gastric cancer screening program | 2,199 (1041/1158) | 35-69 | Antrum (4) | Not specified |
| Corpus (1) | |||||
| Asia | |||||
| China40 | Helicobacter pylori-infected subjects attending gastroenterological clinics | 598 | 18-75 | Antrum (2) | Updated Sydney system |
| Corpus (2) | |||||
| India45 | Symptomatic patients | 355 (263/92) | 13-85 | Not specified | Sydney system |
| Iran46 | General population, 2 districts | 1,011 (494/517) | 40-92 | Incisura (1) | Not specified |
| Antrum (2) | |||||
| Cardia (2) | |||||
| All visible lesions (variable) | |||||
| Japan40 | H. pylori-infected subjects attending gastroenterological clinics | 227 | 51.8 ± 12b | Antrum (2) | Updated Sydney system |
| Corpus (2) | |||||
| Republic of Korea53 | H. pylori-infected individuals who underwent gastroscopic examination for mild epigastric symptoms or routine screening | 181 (122/59) | 50.9 ± 14.9b | Antrum (not specified) | Updated Sydney system |
| Body (not specified) | |||||
| Saudi Arabia54 | Patients being investigated for chronic dyspepsia | 778 (415/363) | 10-100 | Antrum (2) | Not specified |
| Thailand40 | H. pylori-infected subjects attending gastroenterological clinics | 243 | 48.3 ± 14b | Antrum (1) | Updated Sydney system |
| Corpus (1) | |||||
| Turkey56 | Patients admitted to the endoscopic unit | 210 (120/90) | 20-80 | Lesion (3) | Not specified |
| Antrum (1) | |||||
| United Arab Emirates57 | Patients with upper abdominal symptoms that had failed to respond to H2 receptor antagonists or antiacids | 602 (458/144) | 5-70 | Not specified | Not specified |
| Europe | |||||
| Czech Republic42 | Patients with dyspepsia and H. pylori infection | 251 (110/141) | 15-91 | Antrum (2) | Sydney system |
| Corpus (2) | |||||
| Cardia (2) | |||||
| Finland40 | H. pylori-infected subjects attending gastroenterological clinics | 104 | 53.5 ± 10b | Antrum (2) | |
| Corpus (2) | Updated Sydney system | ||||
| Germany40 | H. pylori-infected subjects attending gastroenterological clinics | 250 | 54.1 ± 17b | Antrum (2) | |
| Corpus (2) | Updated Sydney system | ||||
| Ireland47 | Patients presenting upper gastrointestinal symptoms | 324 (168/156) | 59 ±14b | Corpus (not specified) | Not specified |
| Antrum (not specified) | |||||
| Italy48 | Non-ulcerous and untreated patients | 267 (115/152) | 14-84 | Antrum (2) | Not specified |
| Oxyntic mucosa (2) | |||||
| Netherlands40 | H. pylori-infected subjects attending gastroenterological clinics | 263 | 49.9 ±13b | Antrum (2) | Updated Sydney system |
| Portugal40 | H. pylori-infected subjects attending gastroenterological clinics | 221 | 48.8 ±16b | Antrum (2) | Updated Sydney system |
| Corpus (2) | |||||
| Sweden55 | Random sample of participants that had been surveyed using a gastrointestinal symptom questionnaire | 1,000 (490/510) | 20-80 | Cardia (2) | Updated Sydney system |
| Antrum (2) | |||||
| Corpus (2) | |||||
| United Kingdom58 | Patients presenting any degree of dyspepsia and follow-up of those with lesions | 1,753 | > 40c | Not specified | Not specified |
| Oceania | |||||
| Australia39 | Patients with dyspepsia or reflux symptoms | 268 (130/138) | 17-85 | Antrum (3) | Updated Sydney system |
| Body (2) | |||||
| Fundus (2) | |||||
| Incisura (1) | |||||
| New Zealand50 | Patients attending for endoscopy for dyspepsia | 158 (92/66) | 25-80 | Juxtapylorus (2) | Not specified |
| Antrum (2) | |||||
| Body (2) |
M: males; F: females.
Prevalence of intestinal metaplasia according to Helicobacter pylori infection status in 29 countries from 5 continents
| Country, reference | IM prevalence in H. pylori-infected subjects (%) | IM prevalence in H. pylori-uninfected subjects (%) | Overall IM prevalence (%) |
| Africa | |||
| Egypt43 | Not provided | Not provided | Antrum – 24.4 |
| Gambia44 | Not provided | Not provided | 8.9 |
| Nigeria51 | Not provided | Not provided | 9.4 |
| America | |||
| Argentina38 | Not provided | Not provided | 1.8 |
| Colombia41 | 40.7 | 30.8 | 38.8 |
| Mexico49 | Antrum – 21.5 | ||
| Corpus – 9.8 | – | – | |
| Peru52 | Antrum – 29.5 | ||
| Body – 10.2 | |||
| Cardia – 0 | – | – | |
| United States of America53 | Antrum – 15 | ||
| Body – 8.3 | – | – | |
| Venezuela12 | 25.2 | 22.2 | 25 |
| Asia | |||
| China40 | Antrum | – | 33 |
| Corpus – not specified | – | – | |
| India45 | 8.2 | 2.9 | 6.5 |
| Iran46 | Not provided | Not provided | 13 |
| Japan40 | Antrum – | 44 | |
| Corpus – not provided | – | – | |
| Republic of Korea53 | Antrum – 35.1 | ||
| Body – 10.3 | – | – | |
| Saudi Arabia54 | Antrum – 15.5 | Antrum – 14.1 | Antrum – 15.2 |
| Thailand40 | Antrum – 6 | ||
| Corpus – not provided | – | – | |
| Turkey56 | 44.9 | 57.4 | 48.1 |
| United Arab Emirates57 | 34.5 | 14 | 28.9 |
| Europe | |||
| Czech Republic42 | Antrum – 54.6 | ||
| Corpus – 13.9 | |||
| Cardia – 44.2 | – | – | |
| Finland40 | Antrum – 33 | ||
| Corpus – not provided | – | – | |
| Germany40 | Antrum – 22 | ||
| Corpus – not provided | – | – | |
| Ireland47 | Not provided | Not provided | 24.1 |
| Italy48 | Antrum | – | 20.2 |
| Oxyntic mucosa – 6.7 | Antrum – 3.8 | ||
| Oxyntic mucosa – 1.9 | Antrum – 13.8 | ||
| Oxyntic mucosa – 4.9 | |||
| Netherlands40 | Antrum – 26 | – | – |
| Portugal40 | Antrum – 34.0 | ||
| Corpus – not provided | – | – | |
| Sweden55 | Antrum – 23.6 | Antrum – 1.9 | Antrum – 11.3 |
| Corpus – 13.9 | Corpus – 1.9 | Corpus – 7.1 | |
| United Kingdom58 | Not provided | Not provided | 10.5 |
| Oceania | |||
| Australia39 | Antrum – 19.5 | Antrum – 7.7 | Antrum – 12.7 |
| Body – 4.4 | Body – 1.9 | Body – 3 | |
| Fundus – 1.8 | Fundus – 0.6 | Fundus – 1.1 | |
| Incisura – 13.3 | Incisura – 4.5 | Incisura – 8.2 | |
| New Zealand50 | 55 | 26 | 47 |
IM: intestinal metaplasia.
Estimates of tobacco availability in each country (apparent adult tobacco consumption expressed as cigarettes per adult per day) were obtained for 1990-1992 from the Tobacco and Health Report59, or for the nearest available year from the National Tobacco Information Online System (NATIONS) database60 when no data were presented in the previous source (table 3).
Data used in the ecological study (prevalence of intestinal metaplasia (IM) in Helicobacter pylori-infected subjects and tobacco availability)
| Country, reference | IM prevalence in H. pylori-infected subjects (%) | Tobacco availabilitya (cigarettes/day/adult) |
| Africa | ||
| Egypt43 | 24.4b | 3.32 |
| Gambia44 | 11.8c | 1.11d |
| Nigeria51 | 11.1c | 1.01 |
| America | ||
| Argentina38 | 3.0c | 4.41 |
| Colombia41 | 40.7 | 4.79 |
| Mexico49 | 21.5 | 2.66 |
| Peru52 | 29.5 | 0.96 |
| United States of America53 | 15.0 | 7.32 |
| Venezuela12 | 25.2 | 5.26 |
| Asia | ||
| China40 | 33.0 | 5.20 |
| India45 | 8.2 | 3.75 |
| Iran46 | 15.6c | 2.55 |
| Japan40 | 44.0 | 8.88 |
| Republic of Korea53 | 35.1 | 8.25 |
| Saudi Arabia54 | 15.5 | 5.84 |
| Thailand40 | 6.0 | 2.88 |
| Turkey56 | 44.9 | 5.75 |
| Europe | ||
| Czech Republic42 | 54.6 | 6.84 |
| Finland40 | 33.0 | 4.77 |
| Germany40 | 22.0 | 6.46 |
| Ireland47 | 24.1b | 6.63 |
| Italy48 | 20.2 | 5.26 |
| Netherlands40 | 26.0 | 7.73 |
| Portugal40 | 34.0 | 5.51 |
| Sweden55 | 23.6 | 4.25 |
| United Kingdom58 | 10.5b | 6.05 |
| Oceania | ||
| Australia39 | 19.5 | 7.42 |
| New Zealand50 | 55.0 | 6.88 |
IM: intestinal metaplasia.
Data for the years 1990-1992 from the Tobacco and Health report of the World Health Organization (WHO)59, except when otherwise specified.
No information was available regarding the H. pylori infection status and the overall prevalence of IM was used.
Information collected from the National Tobacco Information Online System (NATIONS) database60 (1990-1992).
For the ecological analysis, we considered the prevalence of IM in antrum biopsies of H. pylori-infected subjects. When the site of the biopsy sampling was not specified, we considered prevalence figures, regardless of the location of the lesions. When data on IM prevalence were not presented separately for infected and non-infected individuals38,44,46,51, we assumed that all the cases of IM occurred within the group of infected subjects. When H. pylori status was not analysed in the study, we considered the overall prevalence, regardless of infection status43,47,58. One country had no available data on the consumption of cigarettes in the databases searched and was therefore excluded from the ecological analysis57.
Pearson's correlation coefficients were computed to quantify the association between IM prevalence among the infected and tobacco availability (table 4). Data analysis was performed using STATA», version 9.2.
Association between tobacco availability and prevalence of intestinal metaplasia in Helicobacter pylori-infected individuals according to different inclusion criteria (sensitivity analysis)
| Inclusion criteria | n | Pearson’s correlation coefficient | p |
| All studies | 28 | 0.45 | 0.02 |
| Studies evaluating participants from more than one country | 9 | 0.53 | 0.14 |
| Studies with a sample size of more than 200 participants | 18 | 0.52 | 0.03 |
| Studies providing IM prevalence in the antrum | 17 | 0.33 | 0.19 |
| Studies providing IM prevalence in H. pylori-infected subjects | 21 | 0.36 | 0.11 |
| Studies specifying the use of the updated Sydney system for histological evaluation | 14 | 0.49 | 0.07 |
| Studies collecting 4 or more biopsy samples | 17 | 0.55 | 0.02 |
| Studies involving only asymptomatic subjects | 4 | 0.83 | 0.17 |
| Studies enrolling only individuals aged above 17 | 20 | 0.43 | 0.06 |
| Studies not enrolling only individuals aged above 40 | 24 | 0.42 | 0.04 |
| Studies with a male:female ratio > 1 | 9 | 0.51 | 0.16 |
| Studies published after the year 2000 | 19 | 0.52 | 0.02 |
IM: intestinal metaplasia.
Data on IM prevalence were obtained for 29 countries: 3 African (one from Northern and two from Western Africa), 6 American (one from North, one from Central and four from South American countries), 9 Asian (three from Eastern, 3 from Western and 3 from Southern Asia), 9 European (4 from Northern, 2 from Southern, 2 from Western, and one from Central/Eastern Europe), and 2 from Oceania (Australia and New Zealand) (table 1).
The median number of subjects evaluated in each study was 250, with sample sizes ranging from 45 to 2199. All reports included both males and females, but the number of subjects evaluated according to gender was only available for 18 countries. Among the latter, the median male/female ratio was 1.1, ranging from 0.6 to 3.2. Most studies evaluated samples with a wide agerange (from young adulthood to over 65), but 8 included participants aged below 18 (as specified by the authors or estimated assuming the minimum age as the mean minus 2 standard deviations) and 3 only enrolled participants who were over 40 years old. Twenty-five studies were carried out in a hospital setting, evaluating dyspeptic patients (table 1).
The median number of biopsy specimens colleted was 4, ranging from 2 to 13. The data from 17 studies were based on the Sydney system, while one followed the Whitehead classification, and 11 reports did not specify the criteria used for histological assessment (table 1). Seventeen studies provided information on antrum IM prevalence, while the other only presented data for the frequency of IM, without specifying the location of the lesions found. Seven reports did not present data for IM prevalence by H. pylori infection status (table 2).
The median prevalence of IM among H. pylori-infected individuals was 23.6%, ranging from 3% in Argentina to 55% in New Zealand. In countries presenting a simultaneously high prevalence of infection and low gastric cancer incidence32, IM was also infrequent (Thailand, 6%; India, 8.2%; Nigeria, 11.1%; Gambia, 11.8%; Saudi Arabia, 15.5%; Iran, 15.6%; Egypt, 24.4%) (table 3).
A moderately significant correlation was observed between IM prevalence in infected subjects and tobacco availability (r = 0.45; p = 0.02) (fig. 2). When conducting a sensitivity analysis taking different inclusion criteria into account (table 4), the correlation coefficient point estimates were similar to those observed in the main analysis for most of the subgroups analyzed, with values ranging between 0.42 and 0.55. The only exceptions were in studies providing IM prevalence in the antrum (r = 0.33), studies providing IM prevalence in H. pylori-infected subjects (r = 0.36) and studies involving only asymptomatic subjects (r = 0.83).
DiscussionThis systematic review of published data retrieved information for 29 countries from the 5 continents showing a wide variation in the prevalence of IM among H. pylori-infected subjects. We observed a moderate correlation between IM prevalence among H. pylori-infected subjects and tobacco availability.
The search strategy adopted for the systematic review included only articles published since 1996. These studies were more likely to have followed a standardized approach to the interpretation of gastric biopsies based on the updated Sydney System61. The criteria used to select one study per country were also defined with the aim of selecting the most reliable estimates, with the highest levels of external validity and inter-study comparability of methodologies. Preference was given to articles in which the evaluation of biopsies from patients from different countries was performed by the same authors, which helped to guarantee more homogeneous criteria in the biopsy sampling and interpretation. Studies covering asymptomatic populations within the same country tend to better reflect the prevalence of IM in the region in question and estimates obtained from larger samples are more precise.
The broad inclusion criteria (the oversampling of subjects with conditions known to increase the risk of IM was the main exclusion criterion) allowed an evaluation of studies based on different methodological approaches. Most of them involved dyspeptic and H. pylori-infected patients, which naturally do not reflect the prevalence of IM in the general population. Details of the latter could be obtained by evaluating samples that are not pre-selected according to gastrointestinal symptoms, but such information is only available for a small number of countries. However, our review provides important information on the frequency of IM among H. pylori-infected subjects, which is not expected to differ substantially between dyspeptic and non-dyspeptic subjects, between males and females, or over time.
Even if the evaluated populations are homogeneous, the number and location of biopsy sites may contribute to variability in the prevalence of IM62-64. The studies included in this review showed substantial variation with respect to biopsy sites, or in their description, impairing full comparability between different countries. Here, however, we present data for each specific stomach location and most of the studies cited are based on the results of antrum biopsies. The majority of papers consulted presented information for IM at the antrum, with the greatest prevalence being observed at this location, which facilitated comparison.
Differences in the characteristics of the populations studied with regard to age could compromise comparisons between different countries. Only 3 studies12,40,54 provided the information needed to obtain age-standardized IM prevalence estimates, but most of them included subjects from a wide age-range. All of the studies evaluated both males and females, but the samples do not necessarily reflect the sex ratio within the H. pylori-infected population.
In addition to providing descriptive information on IM prevalence for different countries, the systematic review was also designed to provide data for an ecological analysis, and this was reflected in the characteristics of the search strategy. A more comprehensive search would probably provide information for a few more countries, but we do not believe that the conclusions of our study would be changed since we were able to include a large number of countries from five continents in our analysis.
The advantages and limitations of the ecological design are well-known65 and certain considerations must be made with reference to the data sources and options for analysis.
The information on the frequency of IM had the previously mentioned limitations and some assumptions had to be made in order to include all of the countries in the analysis, since not all the reports presented data in the same format. Seven studies38,43,44,46,47,51,58 did not provide information on the frequency of IM in H. pylori-infected subjects. They were therefore included in the ecological analysis with the assumption either that the overall prevalence of IM was the same as that among the infected (Egypt, Ireland and United Kingdom), or that all IM cases had occurred within the group of infected subjects (Gambia, Nigeria, Argentina and Iran). This seemed a reasonable and conservative option, since prevalence was underestimated in the former group (which includes 2 countries with high cigarette availability and a low or moderate IM prevalence) and overestimated in the latter (which includes three countries with a low IM prevalence and low cigarette availability). If the true figures had been used, the correlation would probably have been stronger. Furthermore, 5 of these studies were conducted in countries with a predictably high prevalence of infection, so the error in our procedure was probably low. In sensitivity analysis, the exclusion of these 7 studies reduced the variability in exposure to tobacco and also the frequency of the outcome, compromising the yield of the ecological study and resulting in a correlation coefficient of 0.36 (23% lower than that observed in the main analysis). However, we computed correlation coefficients for 11 subgroups of studies with methodological characteristics that would favour the validity of our study and obtained similar results for most of the different inclusion criteria considered. The sensitivity analysis supported the robustness of our results, when considering a small number of countries providing prevalence estimates for IM.
The availability of cigarettes derived from production, importation and exportation was used in this study to estimate tobacco use, reflecting both the prevalence and intensity of consumption. We assumed that the proportion of available cigarettes consumed, the underreporting of availability, and the use of other forms of tobacco were similar for all countries.
The tobacco estimates used related to the period 1990-1992 and assumed a lag between exposure and the onset of IM ranging from 5 to 15 years. We have, however, also included in the analysis studies published between 1996 and 2006, which probably refer to surveys performed a few years earlier. The lag time considered in our study is probably of 5 to 10 years on average. Our decision to opt for a time lag as short as 5 years, less than would normally be considered if the outcome was cancer66, was based on the fact that our study estimates the association between smoking and a precancerous lesion, which would be expected to appear several years before the development of gastric cancer3.
The prevalence of IM and cigarette consumption is strongly dependent on age and gender. An age- and sex- standardization of the prevalence of IM and cigarette consumption would have improved our analysis, but unfortunately this was not possible with the data available. It should, however, be acknowledged that we evaluated the frequency of IM among the H. pylori-infected population. This is less likely to be influenced by age than the prevalence of IM in the general population. The effect of the age and gender distributions within the samples evaluated in each study was considered in the sensitivity analysis, showing no major differences in association between IM and smoking when different inclusion criteria were considered relating to the age and gender of the participants in each survey, except in studies that evaluated more women than men.
The United Arab Emirates (UAE) were not considered in the ecological analysis because no information could be obtained for tobacco availability. However, when a value equal to their nearest neighbour (Saudi Arabia) was applied as an estimate for the UAE, the correlations between IM prevalence and smoking remained the same (r = 0.45; p = 0.01).
Our results show an area-level association between exposure to tobacco and IM prevalence in H. pylori-infected subjects, providing ecological support to the hypothesis that smoking is a risk factor for IM among the infected population. This is in agreement with the known increased risk of IM associated with tobacco consumption14-16 and with the results of previous studies that suggest a synergistic effect between smoking and H. pylori infection for stomach cancer32,36. The stronger association between smoking and IM17,67 compared to the relatively low increase in the risk of gastric cancer among smokers10,68 and the low rate of progression from chronic atrophic gastritis to IM observed in Kenya69 suggest that smoking plays a modulating role in this specific step of carcinogenesis.
It would have been interesting to have been able to account for the potential influence of genetic and other environmental factors on regional variations in the prevalence of IM among H. pylori-infected individuals. H. pylori isolates from high-risk stomach cancer populations may differ genetically from those from low-risk areas, but the presence of cagA (the only virulence marker available for a relatively large number of countries) may be of limited value in explaining these apparent «enigmas» as there is also a high prevalence of cagA+ strains in countries with anticipated low frequencies of intestinal metaplasia33,70-73.
Our study shows that IM is relatively infrequent in H. pylori-infected subjects from African and Asian countries. They present a simultaneously high prevalence of infection and low incidence of gastric cancer (Egypt, Gambia, Nigeria, India, Iran, Saudi Arabia and Thailand), which serves to extend the concept of the African and Asian «enigmas» to precancerous lesions. Tobacco availability was positively associated with the prevalence of IM among H. pylori-infected subjects at the area-level.