To review the published evidence of links between radon exposure and central nervous system tumors through a systematic review of the scientific literature.
MethodsWe performed a thorough bibliographic search in Medline (PubMed) and EMBASE. We combined MeSH (Medical Subject Heading) terms and free text. We developed a purpose-designed scale to assess the quality of the included manuscripts.
ResultsWe have included 18 studies, 8 performed on miners, 3 on the general population and 7 on children, and the results have been structured using this classification. The results are inconclusive. An association between radon exposure and central nervous system tumors has been observed in some studies on miners, but not in others. The results observed in the general adult population and in children are also mixed, with some research evincing a statistically significant association and others showing no effect.
ConclusionsWe cannot conclude that there is a relationship between radon exposure and central nervous system tumors. The available studies are extremely heterogeneous in terms of design and populations studied. Further research is needed in this topic, particularly in the general population residing in areas with high levels of radon.
Revisar la evidencia publicada entre la exposición al radón y los tumores del sistema nervioso central a través de una revisión sistemática de la literatura científica.
MétodoSe realiza una revisión sistemática exhaustiva de la literatura científica en Medline (PubMed) y EMBASE, combinando términos MeSH (Medical Subject Heading) y texto libre. Se desarrolla una escala específica para valorar la calidad de los estudios incluidos.
ResultadosSe incluyeron 18 estudios (8 realizados en mineros, 3 en población general y 7 en niños) y los resultados se estructuraron siguiendo esa clasificación. Los resultados son inciertos. Algunos estudios en mineros han observado una asociación entre la exposición a radón y tumores del sistema nervioso central, pero otros no. Los resultados en población general adulta y en niños también son diversos, con algunas investigaciones que encuentran una asociación estadísticamente significativa y otras que no encuentran ningún efecto.
ConclusionesNo puede concluirse que exista una asociación entre la exposición al radón y los tumores del sistema nervioso central. Los estudios disponibles son muy heterogéneos en cuanto al diseño y los sujetos incluidos. Es necesaria más investigación sobre este tema, en particular en población general residente en áreas con elevados niveles de radón.
Central nervous system (CNS) tumors are infrequent. Standardized incidence is 5.9 cases per 100,000 inhabitants in more developed countries for males and 4.4 for females, respectively, according to Globocan 2012.1 Mortality in developed countries is 4.0 and 2.7 per 100,000 inhabitants for males and females, respectively. The incidence rate in Europe for CNS tumors is 28.1 cases per 100,000 inhabitants aged 0-14 (adjusted to the world population).1 CNS tumors can be divided in different histology such as gliomas, neuronal tumors, poorly differentiated tumors, meningiomas and other tumors.
There is very scarce information on the risk factors for these tumors. Its low incidence makes difficult to have more information on their environmental and genetic factors. Some hereditary syndromes increase the risk such as neurofibromatosis, tuberous sclerosis, von Hippel-Lindau syndrome or retinoblastoma.2,3
According to the International Agency for Research on Cancer (IARC), there exists sufficient evidence to classify X and gamma rays exposure as risk factors for CNS tumors.4 This evidence comes mainly from studies based on medical treatment effects. The evidence is very limited with the use of mobile phones.5 Recent studies have observed that there is an association between the use of computerized tomography and brain tumors.6 Other authors suggest that vinyl chloride exposure could increase the risk of CNS tumors.7
Radon 222 is a chemical element pertaining to the noble gases family. Its short-life products Polonium 214 and Polonium 218 release radioactive alpha particles when they are transformed in other products. Radon 222 is the most frequent isotope of radon, comprising 80% of all radon isotopes and it is the most relevant from an epidemiologic point of view. Radon 222 is an odorless, colorless and tasteless gas that is present in the disintegration chain of Uranium 238. Uranium 238 is present in the earth crust rocks and indoor radon concentration depends mainly on the Uranium content of the rocks where a house has been built. Radon is denser than air and therefore radon concentration is usually higher in lower compared to upper floors. Alpha radiation has a poor penetrating capacity and produces damage to the lung epithelium when it impacts on it. There is an established association between radon exposure and lung cancer.8,9 Radon was declared a human carcinogen in 1987 and 1988 by the Environmental Protection Agency10 and by the IARC,4 respectively. It has been included as a risk factor to avoid in new version of the European Code Against Cancer released in October 2014.11 The WHO and EPA state that radon is the first risk factor of lung cancer in never smokers and the second in ever smokers.12 The presence of indoor radon varies depending on the uranium content and, in Spain, it is more frequent in Galicia, certain areas of Castilla y León, Northern Extremadura and Northern of the Comunidad de Madrid.13
Other organs different than the lung, including the kidney and the bone marrow, may receive low doses from radon exposure, as stated in page 14 of the WHO report on indoor radon.12 There are very few studies assessing the relationship between radon and CNS tumors. Radiation dose received by the brain due to radon and its byproducts is much lower than that received by other organs.14 Though definitions of low dose exposure are variable, we could define it as 10mSv, since this is equivalent to one computed tomography in one year. After one year of an average exposure of 200Bq/m3 (Becquerels per cubic meter) by inhalation, the brain would receive between 0.06 and 0.15 mSv whereas the lung in the same situation would receive between 35.8 and 159 mSv. Though there is not a clear pathogenic mechanism, it has been proposed that macrophages might phagocyte small solid particles in the lungs from radon descendants that could reach the CNS through the blood.14 Since ionizing radiations (and radon is an ionizing radiation) are a risk factor for CNS tumors, it is biologically plausible that radon could induce these tumors. Studies analyzing this possible association have shown discrepant results and have been performed mainly in miners and general population.
The aim of this study is to systematically review the existing evidence on the relationship between occupational and residential radon exposure (in adulthood or childhood) with central nervous system tumors.
MethodsWe systematically reviewed the scientific literature. We used Medline (PubMed) and EMBASE for this purpose. We used various combinations of MeSH (Medical Subject Heading) terms combined with free text. Below appears the main bibliographic search used in Medline: ((“Central Nervous System Neoplasms”[Mesh] AND “Brain Neoplasms”[Mesh]) AND “Radon”[Mesh] OR “Radon Daughters”[Mesh]) AND (“humans”[MeSH Terms] AND (English[lang] OR Spanish[lang])).
We completed the search through consulting manually the references of the papers selected to be full-text read. The search period comprised from the first registries in both databases until 30/06/2016. A last update was performed in 21/11/2016. We put especial emphases in the exhaustiveness of the search in order to include all relevant information at the cost of obtaining not relevant information that had to be disregarded. In this case, the manual search of the references retrieved was especially relevant because most of the available papers did not have “central nervous system tumors” or “brain tumors” neither in the title nor in the abstract (or even brain tumors as a MeSH term). Therefore, we decided to look for all miners’ studies analyzing radon and different cancer types to check if in those studies CNS tumors had been also analyzed. A similar complementary approach was also used to locate papers for residential radon and CNS tumors in adults and in children. The potential papers to be included were checked by two reviewers, who decided by consensus if they should be included or not applying certain inclusion and exclusion criteria.
Inclusion and exclusion criteriaWe included studies performed in miners and in general population (adult or pediatric). We did not use restrictions on location. We included studies which assessed radon in air or in water. Regarding language, we included papers written in English or Spanish. We used no restrictions based on sample size and we included all kinds of epidemiological designs. Studies performed in animals were excluded. Those papers analyzing radon exposure on cancer incidence or mortality which did not make a difference on cancer type (i.e. solid cancers) had to be excluded and this was also the case for studies not considering radon as the main exposure (i.e. studies performed in miners that analyzed gamma radiation from uranium or particulate exposure).
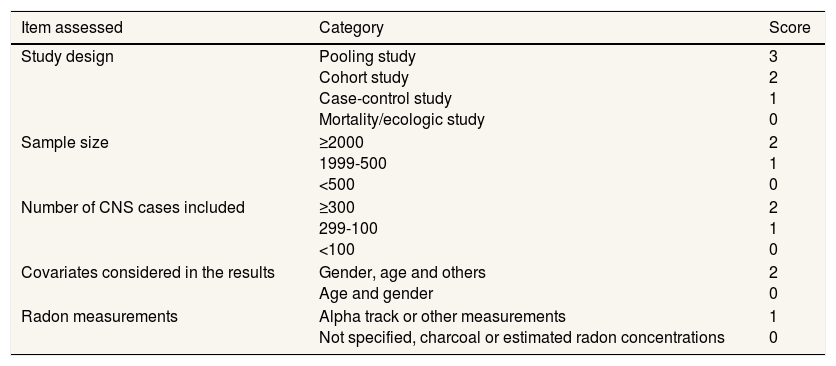
To assess the quality of the included studies we developed a scale that assigned weights to different characteristics of the retrieved studies. Our group has experience in the use of such scales to assess other exposures or health interventions. The detailed scale is shown in Table 1. This scale has been previously used and tested in two recent systematic reviews on radon and lung cancer in never smokers15 and radon and oral and pharyngeal cancers.16 The scale considered five items: study design, sample size, number of cases of CNS tumors included, number of covariates used for adjustment and type of radon measurements. Each of these items had different categories and different scores assigned to them. The scale was easy to use, with a range of 0-10, with those studies with the highest quality obtaining 10 points. The item with the highest weight was study design (up to three points) and the item with the lowest score was type of radon measurements (up to one point). This is a logical weight when assessing the quality of epidemiological studies such as those analyzing radon and cancer types. Two reviewers scored the included studies and any discrepancy was resolved by consensus. The scale assesses indirectly the possibility of bias since the items included give information directly or indirectly on the existence of possible biases. This is the case of the radon device use (information bias), the number of covariates considered (confounding bias) and so on.
Quality scale for the included studies.
| Item assessed | Category | Score |
|---|---|---|
| Study design | Pooling study Cohort study Case-control study Mortality/ecologic study | 3 2 1 0 |
| Sample size | ≥2000 1999-500 <500 | 2 1 0 |
| Number of CNS cases included | ≥300 299-100 <100 | 2 1 0 |
| Covariates considered in the results | Gender, age and others Age and gender | 2 0 |
| Radon measurements | Alpha track or other measurements Not specified, charcoal or estimated radon concentrations | 1 0 |
We divided the results considering the different participants included: miners, general adult population and pediatric population.
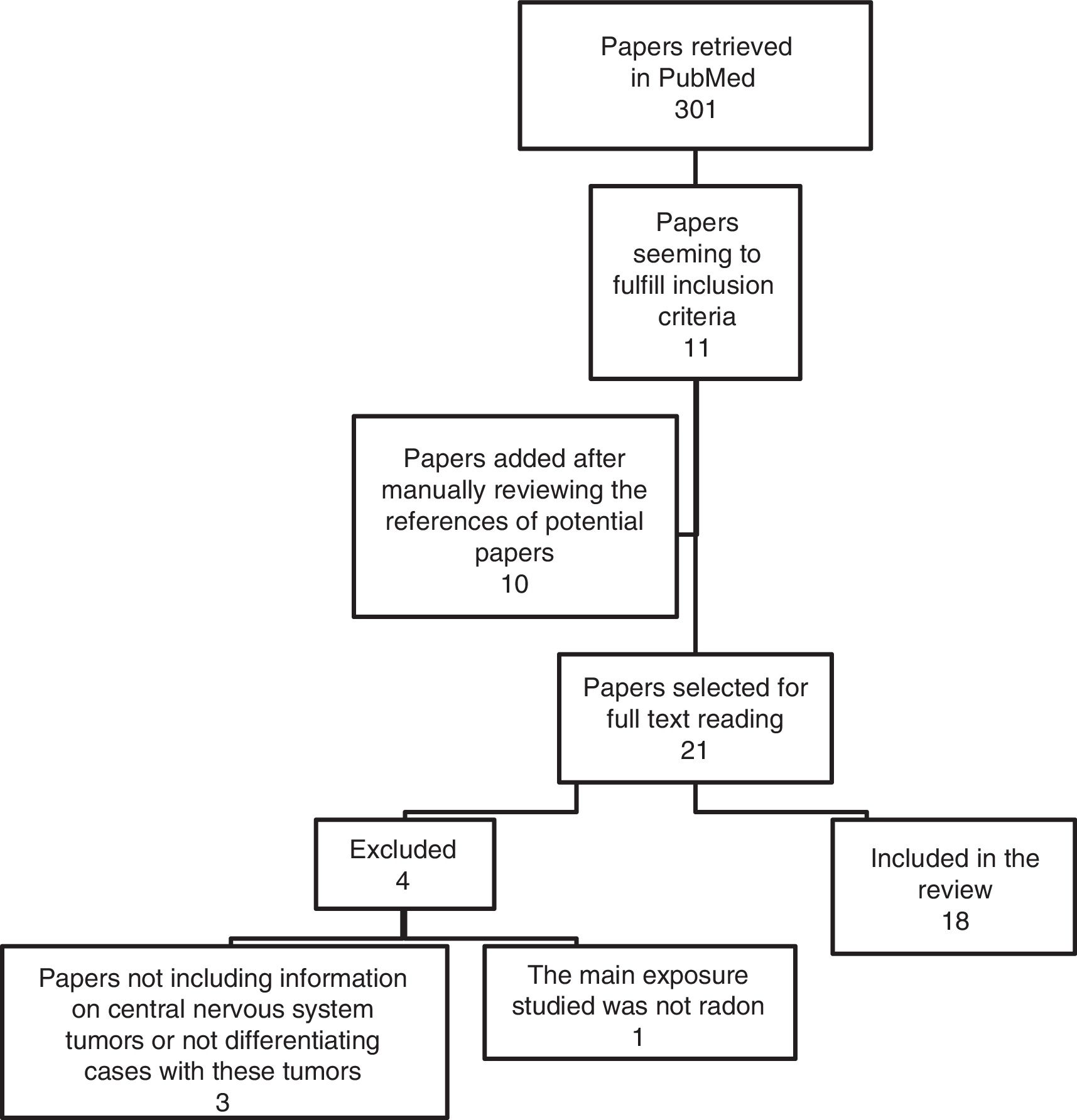
ResultsDescription of the included studiesFigure 1 shows the flowchart of the studies retrieved. 18 papers fulfilled the inclusion criteria. There were different epidemiological designs: cohort studies, case-control, ecologic, hybrid studies and a pooling study performed with data from various cohort studies. Investigations performed in miners included men of median age who were exposed to a different variety of carcinogenic substances in their mining activity. The studies were performed in Europe and North America and the sample size was very heterogenous. Residential radon studies included investigations performed in adults and in pediatric population.
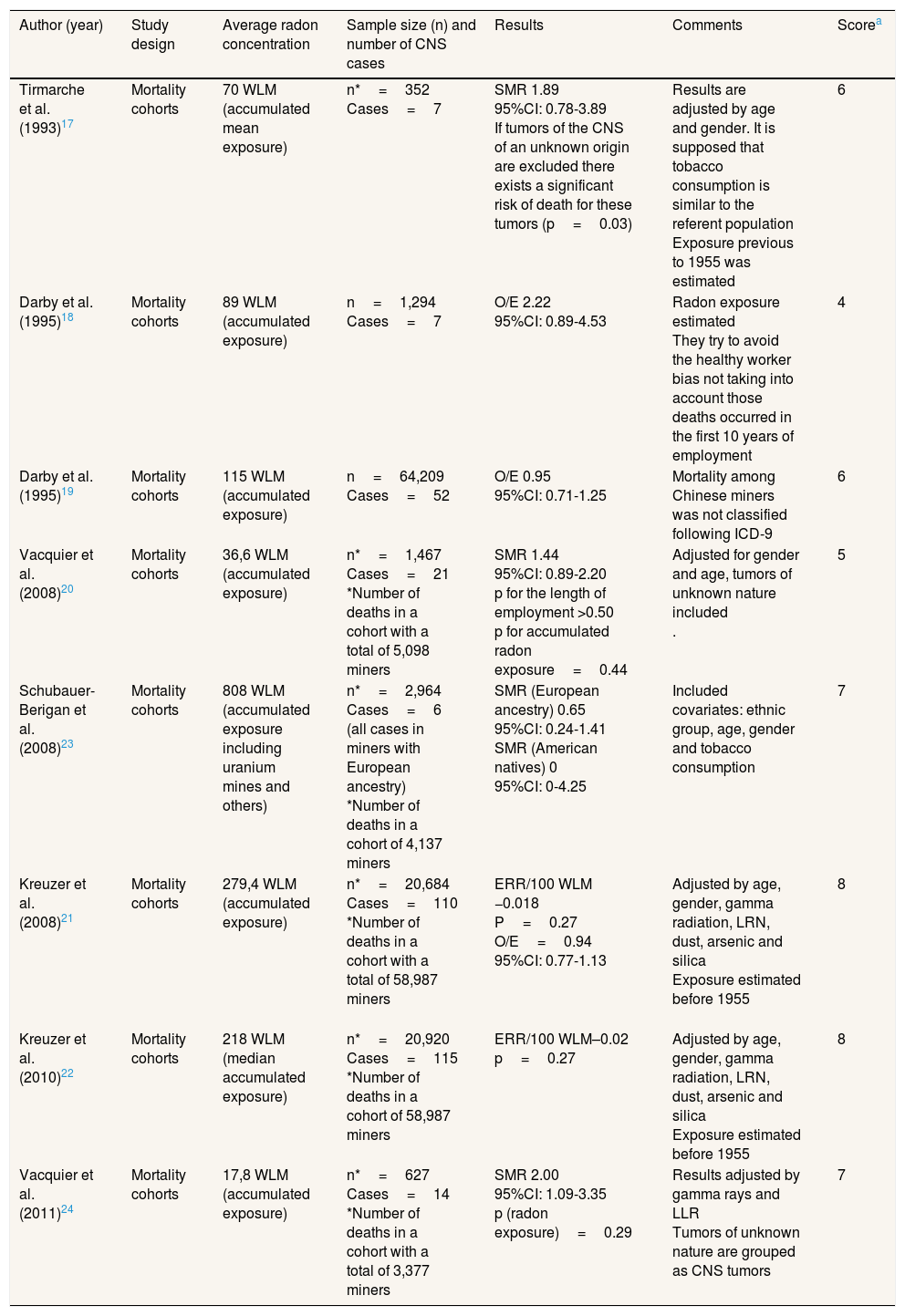
Studies performed in minersWe found eight studies performed in miners.17–24 Radon exposure was measured as working level months (WLM), which is defined as the concentration of short-life descendants per liter of air releasing 1.3×105 MeV of alpha energy. One WLM is approximately equivalent to the dose received by a person who lives during a year in a dwelling with a radon concentration of 225Bq/m3.17 Miners’ studies were performed in France, Germany, United States and Sweden. A description of these studies can be found in Table 2.
Description of the studies performed in miners.
| Author (year) | Study design | Average radon concentration | Sample size (n) and number of CNS cases | Results | Comments | Scorea |
|---|---|---|---|---|---|---|
| Tirmarche et al. (1993)17 | Mortality cohorts | 70 WLM (accumulated mean exposure) | n*=352 Cases=7 | SMR 1.89 95%CI: 0.78-3.89 If tumors of the CNS of an unknown origin are excluded there exists a significant risk of death for these tumors (p=0.03) | Results are adjusted by age and gender. It is supposed that tobacco consumption is similar to the referent population Exposure previous to 1955 was estimated | 6 |
| Darby et al. (1995)18 | Mortality cohorts | 89 WLM (accumulated exposure) | n=1,294 Cases=7 | O/E 2.22 95%CI: 0.89-4.53 | Radon exposure estimated They try to avoid the healthy worker bias not taking into account those deaths occurred in the first 10 years of employment | 4 |
| Darby et al. (1995)19 | Mortality cohorts | 115 WLM (accumulated exposure) | n=64,209 Cases=52 | O/E 0.95 95%CI: 0.71-1.25 | Mortality among Chinese miners was not classified following ICD-9 | 6 |
| Vacquier et al. (2008)20 | Mortality cohorts | 36,6 WLM (accumulated exposure) | n*=1,467 Cases=21 *Number of deaths in a cohort with a total of 5,098 miners | SMR 1.44 95%CI: 0.89-2.20 p for the length of employment >0.50 p for accumulated radon exposure=0.44 | Adjusted for gender and age, tumors of unknown nature included . | 5 |
| Schubauer-Berigan et al. (2008)23 | Mortality cohorts | 808 WLM (accumulated exposure including uranium mines and others) | n*=2,964 Cases=6 (all cases in miners with European ancestry) *Number of deaths in a cohort of 4,137 miners | SMR (European ancestry) 0.65 95%CI: 0.24-1.41 SMR (American natives) 0 95%CI: 0-4.25 | Included covariates: ethnic group, age, gender and tobacco consumption | 7 |
| Kreuzer et al. (2008)21 | Mortality cohorts | 279,4 WLM (accumulated exposure) | n*=20,684 Cases=110 *Number of deaths in a cohort with a total of 58,987 miners | ERR/100 WLM −0.018 P=0.27 O/E=0.94 95%CI: 0.77-1.13 | Adjusted by age, gender, gamma radiation, LRN, dust, arsenic and silica Exposure estimated before 1955 | 8 |
| Kreuzer et al. (2010)22 | Mortality cohorts | 218 WLM (median accumulated exposure) | n*=20,920 Cases=115 *Number of deaths in a cohort of 58,987 miners | ERR/100 WLM–0.02 p=0.27 | Adjusted by age, gender, gamma radiation, LRN, dust, arsenic and silica Exposure estimated before 1955 | 8 |
| Vacquier et al. (2011)24 | Mortality cohorts | 17,8 WLM (accumulated exposure) | n*=627 Cases=14 *Number of deaths in a cohort with a total of 3,377 miners | SMR 2.00 95%CI: 1.09-3.35 p (radon exposure)=0.29 | Results adjusted by gamma rays and LLR Tumors of unknown nature are grouped as CNS tumors | 7 |
95%CI: 95% confidence interval; CNS: central nervous system; ERR: excess of relative risk; ICD-9: International Classification of Diseases-9th revision; LLR/LRN: long lived radionuclides; O/E: observed/expected cases; SMR: standardized mortality ratio; WLM: working level month.
The studies by Tirmarche et al.17 and Vacquier et al.24 observed a positive significant association between radon exposure and CNS tumors. Both were performed in COGEMA (Compagnie Générale des Matières Nuclèaires) and CEA (Comissariat à l’Energie Atomique et aux énergies alternatives) miners in France. Other two studies by Darby et al.19 and Vacquier et al.20 showed marginally significant associations between radon exposure and CNS tumors. The remaining studies did not show any effect for radon exposure with the exception of the study by Schubauer-Berigan et al.,23 which observed an inverse association not statistically significant. Of note, the reviewed studies observed a number of CNS cases that ranged between 623 and 115.22 There was also high variability in radon exposure for the different investigations. The paper by Schubauer-Berigan et al.23 showed the highest radon exposure, 808 Working Level Months compared to the study by Vacquier et al.,24 with 17,8 WLM of average exposure. Most studies showed average exposures of 100-200 WLM and studies with the highest exposure measured in WLM did not show the higher association with CNS tumors. The quality score ranged between 4 and 8, with 6.7 as average score.
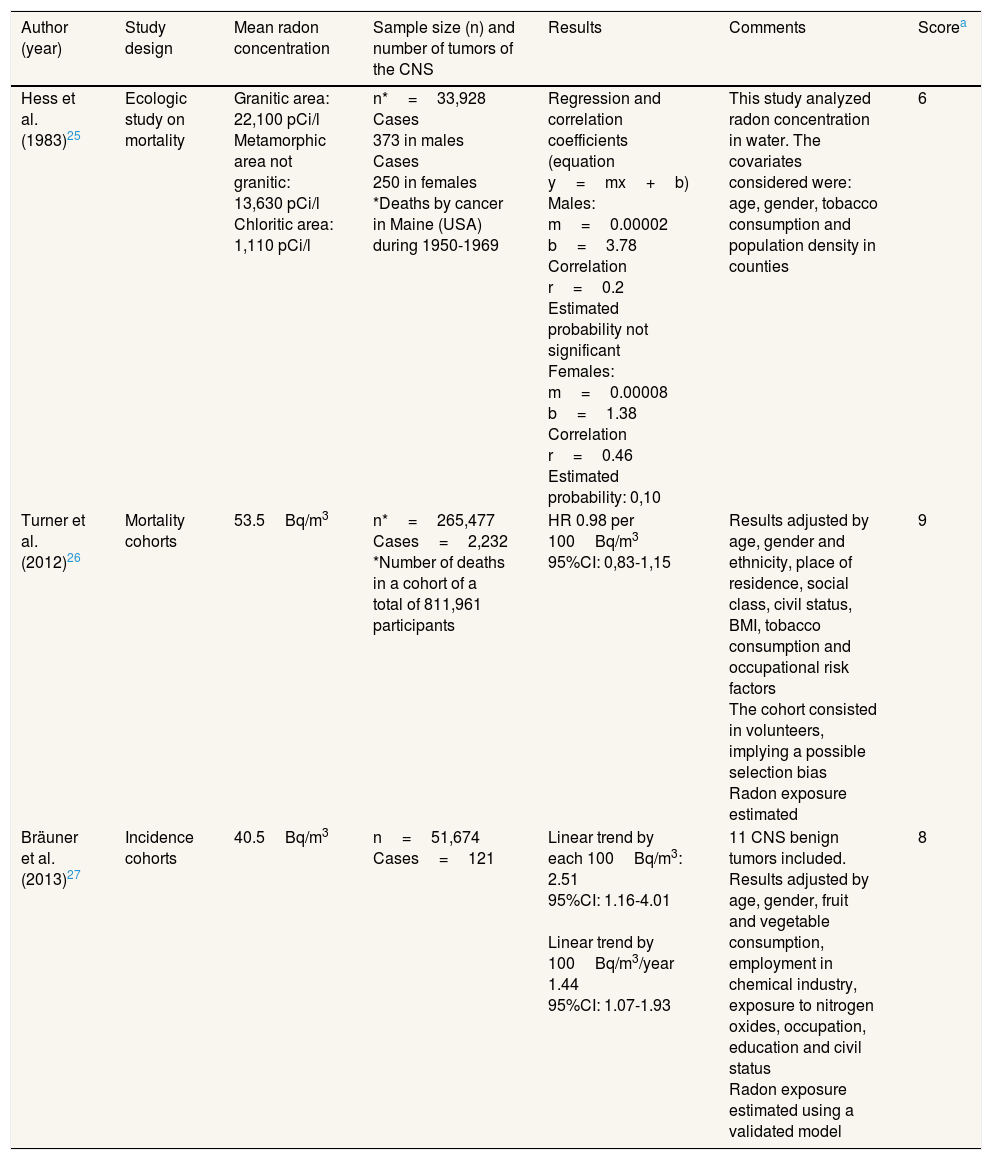
Studies performed between residential radon and central nervous system tumors in adult populationThree studies have been found, published by Hess et al.,25 Turner et al.26 and Bräuner et al.27 Their details can be observed in Table 3. The study by Brauner et al.27 was performed in Denmark and the other two in the United States. The unit of measurement was Bq/m3 and picocuries per liter when radon in water was measured.
Studies performed in general adult population.
| Author (year) | Study design | Mean radon concentration | Sample size (n) and number of tumors of the CNS | Results | Comments | Scorea |
|---|---|---|---|---|---|---|
| Hess et al. (1983)25 | Ecologic study on mortality | Granitic area: 22,100 pCi/l Metamorphic area not granitic: 13,630 pCi/l Chloritic area: 1,110 pCi/l | n*=33,928 Cases 373 in males Cases 250 in females *Deaths by cancer in Maine (USA) during 1950-1969 | Regression and correlation coefficients (equation y=mx+b) Males: m=0.00002 b=3.78 Correlation r=0.2 Estimated probability not significant Females: m=0.00008 b=1.38 Correlation r=0.46 Estimated probability: 0,10 | This study analyzed radon concentration in water. The covariates considered were: age, gender, tobacco consumption and population density in counties | 6 |
| Turner et al. (2012)26 | Mortality cohorts | 53.5Bq/m3 | n*=265,477 Cases=2,232 *Number of deaths in a cohort of a total of 811,961 participants | HR 0.98 per 100Bq/m3 95%CI: 0,83-1,15 | Results adjusted by age, gender and ethnicity, place of residence, social class, civil status, BMI, tobacco consumption and occupational risk factors The cohort consisted in volunteers, implying a possible selection bias Radon exposure estimated | 9 |
| Bräuner et al. (2013)27 | Incidence cohorts | 40.5Bq/m3 | n=51,674 Cases=121 | Linear trend by each 100Bq/m3: 2.51 95%CI: 1.16-4.01 Linear trend by 100Bq/m3/year 1.44 95%CI: 1.07-1.93 | 11 CNS benign tumors included. Results adjusted by age, gender, fruit and vegetable consumption, employment in chemical industry, exposure to nitrogen oxides, occupation, education and civil status Radon exposure estimated using a validated model | 8 |
BMI: body mass index; Bq: Becquerels; 95%CI: 95% confidence interval; CNS: central nervous system; HR: hazard ratio.
The research by Hess et al.25 found an association between radon measured in water and CNS tumors. Bräuner et al.,27 using an incidence cohort, observed a statistically significant effect, with the risk for these tumors increasing linearly (44% per each 100Bq/m3) with radon concentration. This cohort study included 122 cases. Finally, the investigation by Turner et al.,26 consisting in a mortality cohort, did not show any effect with CNS tumors, after including 2,232 cases. The mean radon concentration was very similar in both studies, with 40 and 53.5Bq/m3, respectively,26,27 though the results were discrepant. The scoring of the quality scale ranged from 7 to 9.
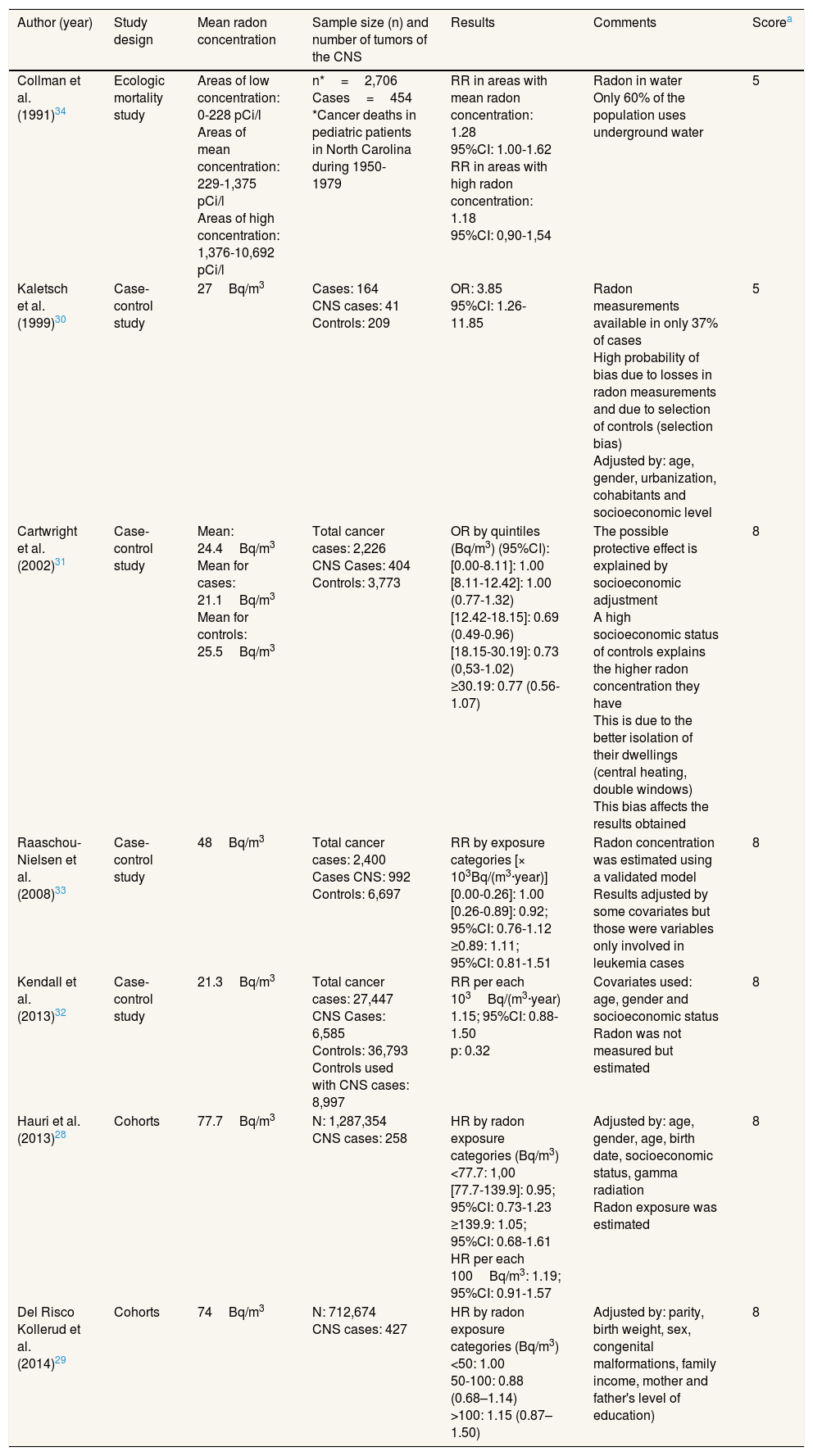
Studies performed between residential radon and central nervous system tumors in pediatric populationThere were seven studies assessing the relationship between radon and CNS tumors in children. Two were cohort studies,28,29 four were case-control studies30–33 and there was one ecologic study.34 The studies were performed in the United States of America, United Kingdom, Denmark, Norway and Switzerland. A detailed description of each study can be observed in Table 4.
Studies performed in children.
| Author (year) | Study design | Mean radon concentration | Sample size (n) and number of tumors of the CNS | Results | Comments | Scorea |
|---|---|---|---|---|---|---|
| Collman et al. (1991)34 | Ecologic mortality study | Areas of low concentration: 0-228 pCi/l Areas of mean concentration: 229-1,375 pCi/l Areas of high concentration: 1,376-10,692 pCi/l | n*=2,706 Cases=454 *Cancer deaths in pediatric patients in North Carolina during 1950-1979 | RR in areas with mean radon concentration: 1.28 95%CI: 1.00-1.62 RR in areas with high radon concentration: 1.18 95%CI: 0,90-1,54 | Radon in water Only 60% of the population uses underground water | 5 |
| Kaletsch et al. (1999)30 | Case-control study | 27Bq/m3 | Cases: 164 CNS cases: 41 Controls: 209 | OR: 3.85 95%CI: 1.26-11.85 | Radon measurements available in only 37% of cases High probability of bias due to losses in radon measurements and due to selection of controls (selection bias) Adjusted by: age, gender, urbanization, cohabitants and socioeconomic level | 5 |
| Cartwright et al. (2002)31 | Case-control study | Mean: 24.4Bq/m3 Mean for cases: 21.1Bq/m3 Mean for controls: 25.5Bq/m3 | Total cancer cases: 2,226 CNS Cases: 404 Controls: 3,773 | OR by quintiles (Bq/m3) (95%CI): [0.00-8.11]: 1.00 [8.11-12.42]: 1.00 (0.77-1.32) [12.42-18.15]: 0.69 (0.49-0.96) [18.15-30.19]: 0.73 (0,53-1.02) ≥30.19: 0.77 (0.56-1.07) | The possible protective effect is explained by socioeconomic adjustment A high socioeconomic status of controls explains the higher radon concentration they have This is due to the better isolation of their dwellings (central heating, double windows) This bias affects the results obtained | 8 |
| Raaschou-Nielsen et al. (2008)33 | Case-control study | 48Bq/m3 | Total cancer cases: 2,400 Cases CNS: 992 Controls: 6,697 | RR by exposure categories [× 103Bq/(m3·year)] [0.00-0.26]: 1.00 [0.26-0.89]: 0.92; 95%CI: 0.76-1.12 ≥0.89: 1.11; 95%CI: 0.81-1.51 | Radon concentration was estimated using a validated model Results adjusted by some covariates but those were variables only involved in leukemia cases | 8 |
| Kendall et al. (2013)32 | Case-control study | 21.3Bq/m3 | Total cancer cases: 27,447 CNS Cases: 6,585 Controls: 36,793 Controls used with CNS cases: 8,997 | RR per each 103Bq/(m3·year) 1.15; 95%CI: 0.88-1.50 p: 0.32 | Covariates used: age, gender and socioeconomic status Radon was not measured but estimated | 8 |
| Hauri et al. (2013)28 | Cohorts | 77.7Bq/m3 | N: 1,287,354 CNS cases: 258 | HR by radon exposure categories (Bq/m3) <77.7: 1,00 [77.7-139.9]: 0.95; 95%CI: 0.73-1.23 ≥139.9: 1.05; 95%CI: 0.68-1.61 HR per each 100Bq/m3: 1.19; 95%CI: 0.91-1.57 | Adjusted by: age, gender, age, birth date, socioeconomic status, gamma radiation Radon exposure was estimated | 8 |
| Del Risco Kollerud et al. (2014)29 | Cohorts | 74Bq/m3 | N: 712,674 CNS cases: 427 | HR by radon exposure categories (Bq/m3) <50: 1.00 50-100: 0.88 (0.68–1.14) >100: 1.15 (0.87–1.50) | Adjusted by: parity, birth weight, sex, congenital malformations, family income, mother and father's level of education) | 8 |
95%CI: 95% confidence interval; CNS: central nervous system; HR: hazard ratio; OR: odds ratio; RR: relative risk.
The observed results were contradictory. Two studies found a positive association between radon and these tumors, but in one of them radon was only measured in water and the other30 had a very low score. Other studies did not find any effect or there was a positive association but without reaching a significant association. The study by Hauri et al.28 showed an association of 1.19 (95% confidence interval [95%CI]: 0.91-1.57) per each 100Bq/m3 of increment in radon concentration and the study by Del Risco Kollerud et al.29 showed an association of 1.13 (0.99–1.28) per each 100Bq/m3 of increment in radon concentration. Mean radon concentrations in case-control and cohort studies were in general quite low. The highest mean radon concentration was observed in the study by Hauri et al.,28 with 77Bq/m3 and the remaining studies had mean radon concentrations below 50Bq/m3. The scoring of the quality scale ranged from 4 to 8 points.
DiscussionTo our knowledge, this is the first systematic review assessing the possible relationship between radon exposure and CNS tumors. No clear association is apparent between radon exposure and the onset of CNS tumors in different settings. This unclear effect is observed when analyzing specifically the results of studies performed in miners, adult general population and children. The methodology of the available studies is highly heterogeneous and also the sample size and the number of cases included in the available studies.
Residential radon has been classified as a lung carcinogen and the scientific evidence is scarce regarding a possible effect on other cancers. Some studies have pointed to a possible association with leukemia,35 skin cancer36 or esophageal cancer.37 The most difficult issue when assessing a possible association with a cancer different than lung cancer is that the effective doses reaching other organs are much lower than that received by the lungs. The studies performed in miners have the advantage that radon exposure is usually higher than that found in residential settings and this aspect makes easier to establish dose-response patterns. Nevertheless, miners’ studies also face the disadvantage that participants are also exposed to other carcinogens such as silica dust, gamma radiation (uranium miners) and other dusts that can be found in mines. All these studies have been performed in males and the number of cases found in these cohorts has been very low in many of them, leading to imprecise estimations. Radon exposure assessment has been variable in these studies. Some of them have used estimations of exposure,18,19 while others have measured individual exposure23 or measured exposure for a part of the follow-up duration.17,20–22,24 Other possible biases might be present in miners’ studies regarding tumor classification. The study by Tirmarche et al.17 used International Classification of Diseases (ICD), 8th version, (that included jointly tumors of unknown nature of the eye, brain or other parts of the CNS). Other studies20,24 employed ICD-9th version (neoplasms of undetermined nature of the brain and benign neoplasms of the CNS). Other studies used other classifications such as ICD-10th version or included only malignant tumors. The investigation by Schubauer-Berigan et al.,23 which observes a negative association between radon and CNS tumors, used the Minor Cause of Death from the National Institute for Occupational Safety and Health Life that includes tumors from the peripheric nervous system. The study by Darby et al.18 did not use the ICD-9th version. These differences difficult the comparison of the results found in miners.
Finally, none of the studies performed in miners had as the main hypothesis to analyze the possible relationship between radon exposure and CNS tumors. Since these tumors have a very low incidence, a cohort study is not probably the best design. A case-control study is a more efficient option. The number of observed deaths is also very low making difficult to obtain any association in these cohorts. If we compare the results observed for overall cancer risk in miners’ studies with the specific result observed for CNS risk, 3 studies17,18,20 have observed a higher risk for CNS tumors while the remaining observed a similar but not a lower risk. It seems therefore that the risk for CNS tumors for radon exposure in miners might be slightly higher than that observed for overall cancer risk in this population. We do not believe that publication bias is present in the relationship between radon and CNS tumors, mainly because this association has been sparsely studied and a specifically designed investigation on this issue should be welcomed by most journals.
There were only three studies assessing residential exposure with CNS tumors in adults. One study measured radon in water and was published decades ago while the other two are very recent and have discrepant results, with no association and a significant dose-response effect. Both studies have a good methodology and the main difference between them is how radon exposure was measured. While in the study by Turner et al.,26 which included a high sample size, mean county radon exposure was assigned to each participant, in the study by Bräuner et al.27 residential radon exposure was assigned individually to each participant after applying a validated model used to estimate radon exposure taking into account different variables.38 The possibility of non-differential exposure misclassification cannot be also excluded when assigning individual residential radon exposure. Another important difference is that the study by Turner et al.26 included participants that were friends or relatives of the selected volunteers, and therefore a potential selection bias could be present, reinforced by the fact that 30% of participants of the cohort were college graduates, and therefore might not represent properly US adult population. Again, there are differences in how the different CNS tumors have been considered as cases, since the included studies have used different classifications. Finally, the Bräuner et al.27 study considered more adjustment variables than the Turner et al.26 study, including diet, occupation, education and exposure to nitrogen oxides.
The studies performed in pediatric population have discrepant results. A proof of the interest in this possible association is the number of available studies, eight, compared with three performed in adults. The main difference with CNS tumors in children vs adults is that the most frequent tumor is meduloblastoma instead of astrocytoma.39 Children are also more sensitive than adults to ionizing radiation because they have a higher number of cells in division compared with adults. An important issue is that the levels of indoor exposure were highly variable in the included studies, i.e. the study by Kendall et al.32 had an average radon exposure of 21Bq/m3 while the study by Hauri et al.28 had an average of 77, close to four times higher. It is important to mention that all studies have been performed in areas that have not been classified as radon prone areas and therefore studies performed in radon prone areas might pose different results. There was also high variability on the design and sample size of the different studies. The investigations with the highest quality were the Swiss and Norwegian cohorts and two case-control studies performed in Denmark and England. None of these investigations directly measured radon exposure in the children's dwellings and radon exposure was estimated using different models. The prediction model for indoor radon exposure in the Hauri et al.28 study had a sensitivity of 0.29-0.31 and a specificity of 0.92. The prediction model by Raaschou-Nielsen et al.33 used in the Danish study correctly predicted 80% of high exposures and 60% of low radon exposures. Of note, the study by Kaletsch et al.,30 which measured radon exposure individually observed a statistically significant odds ratio of CNS tumors of 3.85, being this the highest in all studies. Nevertheless, the study by Cartwright et al.,31 which also measured radon exposure individually, did not find any association. The possibility of a selection bias in the studies by Raaschou-Nielsen et al.,33 Hauri et al.28 and Kendall et al.32 is not present because they used information from the census or from a tumor registry.
The great majority of the included studies have adjusted their results for many covariates. Miners’ studies have adjusted by age, ethnicity, tobacco consumption and gamma rays exposure whereas studies in adult population have adjusted their results by age, gender, tobacco consumption, education and socioeconomic status. Finally, studies performed in children have also adjusted their results by many covariates. These adjustments probably mean that the results observed are not probably due to unmeasured confounding factors and support a low risk of bias in the included studies. This risk is higher in miners’ studies compared to studies performed in adults and in children, a fact reflected in the scoring of each category studies. Most of them did not adjust their results by tobacco consumption or had this information for a limited number of participants. One added limitation is the low incidence of these tumors which produces imprecise estimations in most studies.
The quality of the included studies has been highly variable, with a score between 4 and 9 points. The scoring is particularly heterogeneous for miners and studies performed in children, with studies performed in adults of the general population having a similar quality. Though there are some aspects that we have considered in our quality scale that could be discussed, in our opinion they reflect the main aspects of the included studies such as sample size, number of cases included, study design and number of covariates considered.
The present systematic review has some advantages. To our knowledge, it is the first study reviewing the existing evidence on radon exposure and CNS tumors. We have also developed a quality scale to assess the differences between the studies included and also to compare is validity. The classification of the studies retrieved in occupational, adults and pediatric population, allow us to consider the particular characteristics of these individuals.
This study has some limitations. The main one is that it has not been possible to meta-analyze the included studies, neither globally nor by the subgroups included. This is due to the high heterogeneity of the studies, which are case-control studies, cohort studies or ecological studies. Besides the individual limitations of each study, there is the possibility that we have missed studies published in languages different than English or Spanish. Nevertheless, we are not aware of any study that we have excluded due to language limitations, since we should have found this study in the literature search. A further limitation is that many studies have adjusted their results by few covariates (age and gender mainly) and some of them did not adjust their results for tobacco consumption.
There are few studies that have assessed the relationship between radon exposure and CNS tumors and the results are contradictory. Some rigorous studies have observed statistically significant associations in miners or in general population. Nevertheless, other high quality studies have not observed any association. Two of the studies that found an association were performed in women and children, populations that are not available in miners’ studies. Residential studies are limited because it is difficult to estimate radon exposure. Other problem is that CNS tumors comprise many different diseases and the tumors included are different in some studies.
We can conclude that it is necessary to perform more robust studies, preferably in areas with high radon concentrations and using preferably individual measurements to assess radon exposure. Given the low incidence of CNS tumors and the existence of biological plausibility for a potential effect, more research is needed on this topic.
Exposure to indoor radon is an established risk factor for lung cancer. The literature is not consistent regarding the occurrence of other cancers due to radon exposure. Some studies have shown an association with radon and cancers of the central nervous system while others have not shown any effect.
What does this study add to the literature?This is the first systematic review on this topic. Results are are discrepant, and no conclusion can be established for a possible relationship between radon exposure and central nervous system tumors. Results are also discrepant when different subpopulations are analyzed independently (miners, adults or children). These results warrant more studies particularly in radon prone areas.
Maria-Victoria Zunzunegui.
Authorship contributionsAll authors have contributed equally to the paper's content.
FundingPart of this work has been performed during a Fulbright grant for Senior Researchers awarded to Alberto Ruano-Ravina at Brown University (Providence, Rhode Island, USA) (REF PRX14/00365). This grant is awarded by the US Department of State in collaboration with the Spanish Ministry of Education.
Conflicts of interestNone.