The 3rd International Nursing and Health Sciences Students and Health Care Professionals Conference (INHSP)
More infoThis study aims to compare the average levels of IL-10 with preeclampsia and normotensive in four different tribe populations in South Sulawesi, including Makassar, Bugis, Mandar, and Toraja.
MethodThis research is a cross-sectional study conducted in March–May 2020 in several Health Centre and Hospitals in four areas, namely in the UNHAS Hospital, Jumpandang Baru Health Center, Barabaraya Health Center, Mattirobulu Health Center, Salo Health Center, Lasinrang Pinrang General Hospital, Totoli Health Center, Majene Regional General Hospital, Makale Health Center, Elim General Hospital, Lakipadada Tana Toraja Regional General Hospital. Samples in this study were 88 pregnant women with gestational age >20 weeks which were divided into two groups, namely 44 cases (preeclampsia) and 44 control groups (normotensive). The criteria for the sample in this study are single pregnancy, no systemic disease, and are native Makassar, Bugis, Mandar, and Toraja tribes. Data collected included age, education, occupation, parity, Body Mass Index (BMI), history of preeclampsia. Serum IL-10 levels were examined using the Human Interleukin 10 ELISA Kit.
ResultsThere were significant differences in IL-10 levels in preeclampsia pregnant women in the Makassar, Bugis, Mandar, and Toraja tribes (p=0.020, p<0.05). In contrast to the control group, there was no difference in IL-10 levels in normotensive pregnant women in the Makassar, Bugis, Mandar, and Toraja tribes (p=0.505, p>0.05). The Bugis, Mandar, and Toraja tribes show significant differences in IL-10 levels between preeclampsia pregnant women and normotensive pregnant women with mean rating values of pregnant women who have preeclampsia have lower IL-10 levels than normotensive pregnant women, while the Makassar tribe has valued insignificant difference in IL-10 levels between preeclampsia and normotensive (p=0.309, p>0.05).
ConclusionThere are differences in IL-10 levels in preeclampsia pregnant women in Makassar, Bugis, Mandar, and Toraja tribes. The mean concentration of IL-10 in pregnant women with preeclampsia was significantly lower than in controls.
Preeclampsia is a pregnancy disease defined by hypertension and proteinuria, which is found after 20 weeks of pregnancy, affecting about 2–10% of pregnancies worldwide.1 Preeclampsia is one of the three leading causes of maternal and infant morbidity and mortality.2 Conditions caused by severe complications for both mother and fetus, including preterm labor, IUGR, risk of eclampsia, kidney failure, intracranial hemorrhage, pulmonary edema, stroke, and even death.3,4
Due to preeclampsia, the maternal mortality rate is around 50,000 worldwide every year, with different frequencies in each geographical area. The results of the WHO Global Survey on maternal and perinatal health, as well as some data, found showing the prevalence of preeclampsia in some countries have a difference that is 0.9% in Angola, 3.7% in Uganda, 3.4% in the United States, 3.2% in Argentina, 8.9% in Brazil, 3.3% in Australia, 12% in Bangladesh, 3.2% in India, 1.2% in Vietnam, 5.6% in the Philippines, 4.7% in Thailand, the incidence of preeclampsia in Indonesia alone is 128,273/year or around 5.3%. The prevalence of preeclampsia in developed countries is 1.3–6%, whereas, in developing countries, it is 1.8–18%. From the data report, geographical location and ethnicity can be a risk factors for the development of preeclampsia.5–8
Several models have been proposed to explain the pathogenesis of preeclampsia. One well-accepted hypothesis is that defects in early placentation, especially the invasion of endovascular trophoblasts, lead to the development of an inadequate uteroplacental blood supply capable of inducing clinical manifestations of the disease.9,10 Abnormal maternal immune responses result in an increase in excessive systemic inflammatory responses during pregnancy. Previous studies reported that changes in cytokine concentrations might play an important role in trophoblast invasion and endothelial dysfunction in preeclampsia.11 Normal pregnancy is characterized by T-helper 2 (Th2) predominance over T-helper 1 (Th1). Th1 cells produce pro-inflammatory cytokines such as IL-6, IL-2, IFN-γ, and TNF-α. Th2 cells produce anti-inflammatory cytokines such as IL-10, IL-4, IL-5, and IL-13 and generate humoral immunity. However, preeclampsia has been shown to have higher levels of Th1 (pro-inflammatory) products and lower levels of Th2 (anti-inflammatory) products than normal pregnancies in blood serum.12,13
IL-10 is a cytokine that has an important role in pregnancy regulating the maternal immune response, has anti-inflammatory effects, and affects vascular at the maternal–fetal interface.14 Decreased production of IL-10 can trigger poor placentation and anti-angiogenesis activation, as well as hypoxia of placental tissue, which contributes to the development of preeclampsia.12 Several previous research studies have analyzed the relationship between IL-10 levels in women with preeclampsia. Decreased IL-10 production can cause poor placentation and induction of vasoactive anti-angiogenic factors in which tissue and serum placenta preeclampsia have reduced levels of IL-10.15 Several experimental studies collected showed IL-10 deficiency in placental tissue and serum samples of preeclampsia in women causing placental tissue hypoxia and anti-angiogenic activation.16
Indonesia is a country that has tribe, ethnic, and cultural diversity. One of them is located in South Sulawesi, which residents visit are Bugis, Makassar, Mandar, and Toraja. Unfortunately, no one has compared IL-10 levels in each tribe/ethnic group in Indonesia so far. We aim to compare serum IL-10 levels in Makassar, Bugis, Mandar, and Toraja tribes in South Sulawesi Province, Indonesia.
MethodsThis study was carried out after obtaining ethical approval from the Ethics Committee of the Hasanuddin University Medical Faculty.
Study designThis research is a cross-sectional study involving 88 pregnant women recruited between March and May 2020 from several Puskesmas and Hospitals in four regions in South Sulawesi, namely Makassar, Pinrang, Majene, and Toraja, namely in the UNHAS Hospital, Jumpandang Baru Health Centre, Barabaraya Health Centre, Mattirobulu Health Centre, Salo Health Centre, Lasinrang Pinrang General Hospital, Totoli Health Centre, Majene Regional General Hospital, Makale Health Centre, Elim General Hospital, Lakipadada Tana Toraja Regional General Hospital. Out of 88 pregnant women, 44 people diagnosed with preeclampsia were included as a case group consisting of 11 Makassar tribes, 11 Bugis tribes, 11 Mandar tribes, and 11 Toraja tribes. The diagnostic criteria are increased systolic blood pressure ≥140 and diastolic blood ≥90mmHg and positive proteinuria (+) with dipstick after gestational age>20 weeks. Patients with preexisting hypertension, diabetes, chronic kidney, hepatic or vascular disease were excluded from the study. The controls in this study were pregnant women with the same gestational age as the case group that is >20 weeks, absence of symptoms of elevated blood pressure, proteinuria, and absence of the predisposing diseases listed above. PE as many as 44 people divided into 11 people each tribe. This study has obtained ethical recommendations with protocol number UH19121051.
Data types and sourcesData collected from the sample are demographic data, including age, education, occupation, parity, Body Mass Index (BMI), and history of preeclampsia.
Sample collection and preparationData related to demographic and obstetric history samples were collected using a questionnaire through direct interviews with respondents. Blood samples of 3ml of venous blood were collected in a vacutainer tube without additives from each sample, and the researchers were assisted by laboratory staff at the hospital and the research center. The blood sample is then centrifuged to form serum. The serum is separated and then transferred into a new tube and stored in the refrigerator at −20°C. After all the samples were collected, the IL-10 level was examined using the Human Interleukin-10 ELISA Kit Hasanuddin University Medical Research Center (HUM-RC) Laboratory.
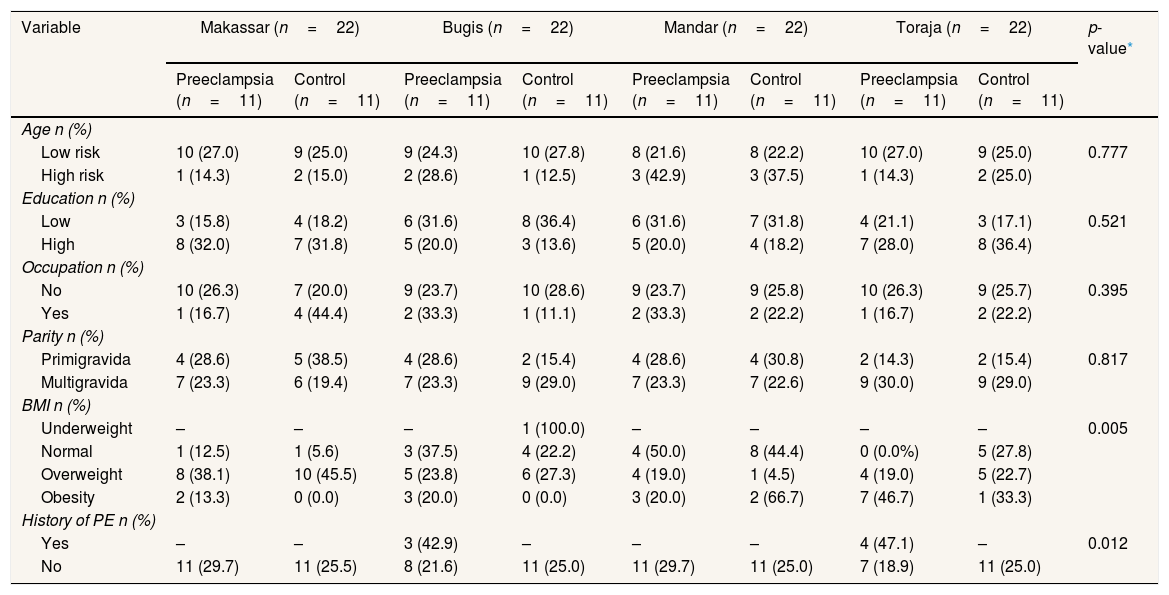
ResultData shows the characteristics of age, education, occupation, parity, Body Mass Index (BMI), and history of preeclampsia among preeclampsia and normotensive groups in Makassar, Bugis, Mandar, and Toraja tribes (Table 1).
Demographic, clinical, and obstetric characteristics of respondents.
| Variable | Makassar (n=22) | Bugis (n=22) | Mandar (n=22) | Toraja (n=22) | p-value* | ||||
|---|---|---|---|---|---|---|---|---|---|
| Preeclampsia (n=11) | Control (n=11) | Preeclampsia (n=11) | Control (n=11) | Preeclampsia (n=11) | Control (n=11) | Preeclampsia (n=11) | Control (n=11) | ||
| Age n (%) | |||||||||
| Low risk | 10 (27.0) | 9 (25.0) | 9 (24.3) | 10 (27.8) | 8 (21.6) | 8 (22.2) | 10 (27.0) | 9 (25.0) | 0.777 |
| High risk | 1 (14.3) | 2 (15.0) | 2 (28.6) | 1 (12.5) | 3 (42.9) | 3 (37.5) | 1 (14.3) | 2 (25.0) | |
| Education n (%) | |||||||||
| Low | 3 (15.8) | 4 (18.2) | 6 (31.6) | 8 (36.4) | 6 (31.6) | 7 (31.8) | 4 (21.1) | 3 (17.1) | 0.521 |
| High | 8 (32.0) | 7 (31.8) | 5 (20.0) | 3 (13.6) | 5 (20.0) | 4 (18.2) | 7 (28.0) | 8 (36.4) | |
| Occupation n (%) | |||||||||
| No | 10 (26.3) | 7 (20.0) | 9 (23.7) | 10 (28.6) | 9 (23.7) | 9 (25.8) | 10 (26.3) | 9 (25.7) | 0.395 |
| Yes | 1 (16.7) | 4 (44.4) | 2 (33.3) | 1 (11.1) | 2 (33.3) | 2 (22.2) | 1 (16.7) | 2 (22.2) | |
| Parity n (%) | |||||||||
| Primigravida | 4 (28.6) | 5 (38.5) | 4 (28.6) | 2 (15.4) | 4 (28.6) | 4 (30.8) | 2 (14.3) | 2 (15.4) | 0.817 |
| Multigravida | 7 (23.3) | 6 (19.4) | 7 (23.3) | 9 (29.0) | 7 (23.3) | 7 (22.6) | 9 (30.0) | 9 (29.0) | |
| BMI n (%) | |||||||||
| Underweight | – | – | – | 1 (100.0) | – | – | – | – | 0.005 |
| Normal | 1 (12.5) | 1 (5.6) | 3 (37.5) | 4 (22.2) | 4 (50.0) | 8 (44.4) | 0 (0.0%) | 5 (27.8) | |
| Overweight | 8 (38.1) | 10 (45.5) | 5 (23.8) | 6 (27.3) | 4 (19.0) | 1 (4.5) | 4 (19.0) | 5 (22.7) | |
| Obesity | 2 (13.3) | 0 (0.0) | 3 (20.0) | 0 (0.0) | 3 (20.0) | 2 (66.7) | 7 (46.7) | 1 (33.3) | |
| History of PE n (%) | |||||||||
| Yes | – | – | 3 (42.9) | – | – | – | 4 (47.1) | – | 0.012 |
| No | 11 (29.7) | 11 (25.5) | 8 (21.6) | 11 (25.0) | 11 (29.7) | 11 (25.0) | 7 (18.9) | 11 (25.0) | |
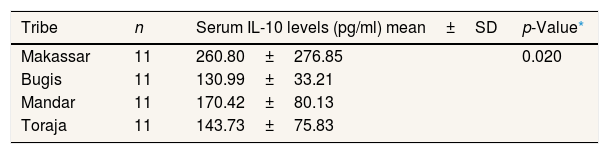
In the preeclampsia group, the analysis results showed a difference in the average IL-10 level in women who experienced preeclampsia in the Makassar, Bugis, Mandar, and Toraja tribes (p=0.020, p<0.05). This result is supported by the average value indicating that women pregnant with pre-eclampsia in Makassar had the highest IL-10 level compared to other ethnic groups, while preeclampsia women in the Bugis tribe had the lowest IL-10 level compared to other ethnic groups (Table 2).
Mean serum IL-10 levels in pregnant women with preeclampsia in the Bugis, Makassar, Mandar, and Toraja tribes (n=44).
| Tribe | n | Serum IL-10 levels (pg/ml) mean±SD | p-Value* |
|---|---|---|---|
| Makassar | 11 | 260.80±276.85 | 0.020 |
| Bugis | 11 | 130.99±33.21 | |
| Mandar | 11 | 170.42±80.13 | |
| Toraja | 11 | 143.73±75.83 |
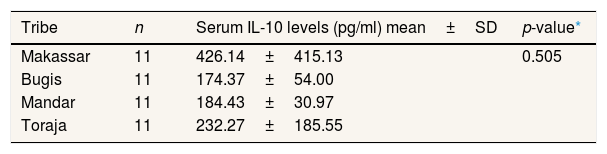
In the normotensive group, the results of the analysis showed no difference in IL-10 levels in normotensive pregnant women in the Makassar, Bugis, Mandar, and Toraja tribes with p=0.505 (p>0.05) (Table 3).
Mean serum IL-10 levels in normotensive pregnant women in the Bugis, Makassar, Mandar, and Toraja tribes (n=44).
| Tribe | n | Serum IL-10 levels (pg/ml) mean±SD | p-value* |
|---|---|---|---|
| Makassar | 11 | 426.14±415.13 | 0.505 |
| Bugis | 11 | 174.37±54.00 | |
| Mandar | 11 | 184.43±30.97 | |
| Toraja | 11 | 232.27±185.55 |
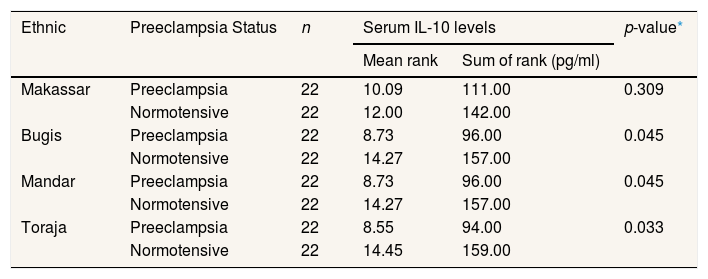
The results of the analysis of the combination of preeclampsia and normotensive groups in each tribe showed significant differences in IL-10 levels between preeclampsia pregnant women and normotensive pregnant women in the Bugis tribe (p=0.045, p<0.05), Mandar (p=0.045, p<0.05), and Toraja (p=0.033, p<0.05). In comparison, the Makassar tribe did not show differences in IL-10 levels between preeclampsia and normotensive pregnant women (p=0.309, p>0.05) (Table 4).
Differences in IL-10 levels between preeclampsia and normotensive pregnant women in the Bugis, Makassar, Mandar, and Toraja tribes (n=88).
| Ethnic | Preeclampsia Status | n | Serum IL-10 levels | p-value* | |
|---|---|---|---|---|---|
| Mean rank | Sum of rank (pg/ml) | ||||
| Makassar | Preeclampsia | 22 | 10.09 | 111.00 | 0.309 |
| Normotensive | 22 | 12.00 | 142.00 | ||
| Bugis | Preeclampsia | 22 | 8.73 | 96.00 | 0.045 |
| Normotensive | 22 | 14.27 | 157.00 | ||
| Mandar | Preeclampsia | 22 | 8.73 | 96.00 | 0.045 |
| Normotensive | 22 | 14.27 | 157.00 | ||
| Toraja | Preeclampsia | 22 | 8.55 | 94.00 | 0.033 |
| Normotensive | 22 | 14.45 | 159.00 | ||
The statistical tests in Table I show that age, education, occupation, and parity did not differ significantly either in the normotensive and preeclampsia groups (p>0.05). Meanwhile, history of preeclampsia (p=0.012, p<0.05), and Body Mass Index (BMI) (p=0.005, p<0.05) showed a significant difference. Women with a first pregnancy have preeclampsia, so the woman has a seven times higher risk of having preeclampsia in the second pregnancy. Similarly, if the preeclampsia occurs in the second pregnancy, the risk of recurring in the third pregnancy is also high seven times.17 Overweight and obesity are strongly associated with a higher risk of gestational diabetes, preeclampsia, cesarean delivery, and macrosomia.18 Obesity has been associated with a 2–4 fold increased risk of preeclampsia in different populations.19 Obesity is characterized by the expansion of adipose tissue (fat) in the body. Furthermore, maternal obesity and circulating factors, such as non-esterified fatty acids, may contribute to excess lipid accumulation in the placenta.20,21 This can interfere with placental development, including trophoblast invasion and angiogenesis and nutrient transport between mother and fetus, resulting in increased oxidative stress and inflammation at the maternal–fetal interface.20 These placental injuries often characterize PE pregnancies. The connection between maternal obesity and PE is hypothesized to involve immune cells within the mother's adipose tissue and in the placenta contributing to impaired placentation.22
The findings in the study are that there are differences in IL-10 levels between Makassar, Bugis, Mandar, and Toraja tribes who experience preeclampsia. This result is supported by the different mean values of each ethnic group in Table 2. The Makassar tribe had an IL-10 level of 260.80pg/ml, in the Bugis group of 130.99pg/ml, the Mandar tribe group at 170.42pg/ml, and Toraja group 143.73pg/ml. This shows that preeclampsia pregnant women in Makassar have the highest IL-10 levels compared to other tribes, while preeclampsia pregnant women in the Bugis tribe have the lowest IL-10 levels compared to other tribes. Whereas for normotensive pregnant women from the results of statistical tests, each ethnic group showed no difference in IL-10 levels in normotensive pregnant women in Makassar, Bugis, Mandar, and Toraja tribes. In Table 3, average IL-10 test results in the Makassar tribe of 426.14pg/ml, the Bugis tribe of 174.37pg/ml, and the Mandar tribe of 184.43pg/ml, and the Toraja tribe of 232.27pg/ml. Even so, the mean value shows that normotensive pregnant women in Makassar have higher IL-10 levels compared to other ethnic groups, while normotensive pregnant women in Bugis tribe have IL-10 levels, which are slightly lower than other ethnic groups
Samples of preeclampsia and normotensive in each ethnic group were combined for further analysis. The test results in Table 4 showed significant differences in IL-10 levels between preeclampsia pregnant women and normotensive pregnant women in the Bugis, Mandar, and Toraja tribes. This is supported by the average rating of pregnant women in the Bugis, Mandar, and Toraja tribes who have preeclampsia having lower IL-10 levels compared to normotensive pregnant women. The Bugis and Mandar tribes have the same rank value of IL-10 levels of 96.00pg/ml for the preeclampsia group and 157.00pg/ml for the normotensive group. The Toraja tribe has a rating value of the amount of IL-10 levels in the preeclampsia group that is 94.00pg/ml during normotensive 159pg/ml. If seen in the Makassar tribe, there is no difference in IL-10 levels between preeclampsia pregnant women and normotensive pregnant women. However, in Makassar, the pre-eclampsia level was slightly lower at 111.00pg/ml compared to normotensive pregnant women at 142.00pg/ml.
One hypothesis about preeclampsia is the occurrence of an immune response maladaptation and failure of trophoblast invasion. During the pregnancy process, immune system adaptation from the maternal side will be needed to “semi-allograft.”16 An increase in the maternal excess inflammatory response in response to foreign antigens from the fetus leads to a series of events in the form of inadequate trophoblast invasion, failure of spiral arterial remodeling, placental ischemia, and release of pro-inflammatory cytokines that can cause clinical manifestations of the disease in pregnancy, one of which is preeclampsia.14
In a normal pregnancy, there is a shift in the Th1/Th2 immune balance towards a Th2 type immune response that protects the fetus from a Th1 (cytotoxic) immune response that can harm the fetus with its products such as TNF-α, IL-2, and interferon-γ.23 Conversely, in preeclampsia, a maternal immune system maladaptation to the fetoplacental occurs where the level of Th1 (pro-inflammatory) product is higher, and the level of Th2 (anti-inflammatory) product such as IL-10 is lower compared to normal pregnancy in blood serum.24
Results Research studies conducted in Baghdad found that the average concentration of plasma IL-10 levels in normal pregnant women was significantly higher (p<0.05) than in all preeclampsia groups.11 In a study conducted by Kalkunte et al.,15 reduced production of IL-10 can cause poor placentation and induction of vasoactive anti-angiogenic factors. This is in accordance with the evaluation of tissue and serum placenta preeclampsia obtained a reduction in IL-10 levels. The Meta-analysis study regarding IL-10 in pregnancy showed several experimental studies that collected that IL-10 deficiency can cause hypoxia of placental tissue and anti-angiogenic activation. This idea is then supported by data from human studies showing reduced IL-10 production in placental tissue and serum samples from preeclampsia women.16 However, not all studies have resulted in a decrease in IL-10, a research study conducted in Sudan showed a significantly high increase in IL-10 in preeclampsia compared with controls (p=0.002),25 whereas other research in Iran showed no significant IL-10 difference between preeclampsia and normal pregnancy.26
The variability in the differences in IL-10 results in the preeclampsia and normotensive groups obtained in some previous good studies explained that it was likely to be influenced by the research design used, including ethnic or ethnic differences in respondents, the method which is used to detect IL-10, and the gestational age at which the sample was obtained. In addition, the short half-life of IL-10 can also contribute to research results.12
Differences in levels between tribes or ethnicities are influenced not only by fetal–maternal gene reactions and environmental influences but can also be due to differences in alleles and genotypic distribution, which in turn results in a different distribution of cytokine levels and may be related to different predispositions and/or outcomes in clinical preeclampsia.27 Several research studies show that IL-10 production is also genetically determined, and controlled from the level of transcription, perhaps through the order of regulation in the promoter area, so that the IL-10 relationship to the development of preeclampsia also differs in each ethnic group, such as in the Chinese, Tunisian, European, Iranian and white and black groups.1,3,28
Our findings underline the need for further investigation of biological mechanisms for these findings that can help understand the pathophysiological differences underlying conditions between racial/ethnic groups and preeclampsia and help reduce health disparities and improve maternal and infant health and well-being. The study of a larger population is needed to validate the above findings.
ConclusionThere are differences in IL-10 levels in the preeclampsia group in four tribes in South Sulawesi. The mean concentration of IL-10 in pregnant women with preeclampsia was significantly lower than in controls. In this study, pregnant women with preeclampsia may show impaired immunological tolerance, trophoblast invasion, and impaired placentation.
Conflicts of interestThe authors declare no conflict of interest.
Peer-review under responsibility of the scientific committee of the 3rd International Nursing, Health Science Students & Health Care Professionals Conference. Full-text and the content of it is under responsibility of authors of the article.