To present surveillance data on advanced disease (AD) and late presentation (LP) of HIV in Spain and their determinants.
MethodsWe included all new HIV diagnoses notified by the autonomous regions that consistently reported such cases throughout the period 2007–2011. Coverage was 54% of the total Spanish population. Data sources consisted of clinicians, laboratories and medical records. AD was defined as the presence of a CD4 cell count <200cells/μL in the first test after HIV diagnosis, while LP was defined as the presence of a CD4 cell count <350cells/μL after HIV diagnosis. Odds ratios and their 95% confidence intervals (OR, 95% CI) were used as the measure of association. Logistic regressions were fit to identify predictors of AD and LP.
ResultsA total of 13,021 new HIV diagnoses were included. Among these, data on the outcome variable were available in 87.7%. The median CD4 count at presentation was 363 (interquartile range, 161–565). Overall, 3356 (29.4%) patients met the definition of AD and 5494 (48.1%) were classified as LP. Both AD and LP increased with age and were associated with male sex and infection through drug use or heterosexual contact. All immigrants except western Europeans were more prone to AD and LP. Multivariate models disaggregated by sex showed that the effect of age and region of origin was weaker in women than in men.
ConclusionsDespite universal health care coverage in Spain, men, immigrants and people infected through drug use or heterosexual contact seem to be experiencing difficulties in gaining timely access to HIV care.
Se presentan los datos de vigilancia sobre enfermedad avanzada y presentación tardía de los nuevos diagnósticos de VIH en España, y sus determinantes.
MétodosSe incluyeron todos los nuevos diagnósticos de VIH de 2007–2011 en el ámbito de las comunidades autónomas que notificaron de forma constante durante todo el periodo (54% de la población española). La fuente de información fueron clínicos y laboratorios. Se definió como enfermedad avanzada un recuento < 200 linfocitos CD4/μl en la primera determinación tras el diagnóstico, y como presentación tardía < 350 linfocitos CD4/μl. Se usaron la odds ratio y su intervalo de confianza del 95% como medida de asociación. Para el análisis multivariado de los factores asociados a enfermedad avanzada y presentación tardía se ajustó un modelo de regresión logística.
ResultadosSe incluyeron 13.021 nuevos diagnósticos, de los cuales el 87,7% tenía información de la variable de estudio. La mediana de CD4 fue de 363 (rango intercuartílico: 161–565). Durante el periodo, 3.356 pacientes (29,4%) cumplían la definición de enfermedad avanzada y 5.494 (48.1%) se clasificaron como presentación tardía. Tanto la enfermedad avanzada como la presentación tardía aumentaban con la edad, se asociaban al sexo masculino y a la transmisión a través del uso de drogas inyectadas o heterosexual. Ser inmigrante de cualquier origen, excepto de Europa Occidental, se asociaba a enfermedad avanzada y presentación tardía. Desagregando por sexo, el efecto de la edad y de la región de origen fue más débil en las mujeres que en los hombres.
ConclusiónA pesar de la cobertura universal en España, los hombres, los inmigrantes, los usuarios de drogas inyectadas y las personas infectadas por relaciones heterosexuales parecen tener más dificultad para acceder al seguimiento clínico.
Early HIV diagnosis and treatment are essential in the control of the HIV epidemic worldwide. HIV-infected patients presenting late in the course of their infection have higher rates of morbidity and mortality and require more healthcare resources than those presenting earlier.1,2 Furthermore, HIV diagnosis motivates infected people to adopt measures to reduce the risk of infecting others, and treatment with highly active antiretroviral therapy (HAART) has been shown to greatly reduce HIV transmission.3–5
Different criteria have been used in the literature to define diagnostic delay and late presentation among HIV-infected patients, which generally include the level of CD4 cells and the presence/absence of AIDS-defining diseases.6 Recently, European groups have proposed that “late presentation” be defined as the presence of an AIDS condition or CD4 cell count <350cells/μL at presentation for care, leaving the term “advanced disease” to describe the presence of either an AIDS condition or a CD4 cell count <200cells/μL at presentation.7,8
Late presentation remains a major challenge for the control of HIV in Europe, and reducing the time between infection and entry to care is a priority for the region.9–11 Measuring CD4 cells at presentation for care is routine practice in most countries – in particular in the Western part of the region. But, in 2011, only 56% of new HIV diagnoses reported to the European Centre for Disease Control (ECDC) from the EU countries had CD4 data.12
In Spain, epidemiological surveillance on new HIV diagnosis is performed through the Sistema de Información sobre Nuevos Diagnósticos de VIH (SINIVIH in its Spanish acronym) which collects epidemiological and clinical data on people newly diagnosed with HIV. This system, expected to be implemented countrywide in the near future, currently includes data from 17 out of 19 of Spain's autonomous regions (AR). CD4 cell count at presentation is collected, and the completion of the variable is good.
Previously published SINIVIH data showed that almost 40% of new HIV diagnoses during the period 2003–2007 had <200 CD4, and that this situation was more likely to occur in men, people over 30 years, intravenous drug users (IDU) and heterosexuals; factors associated with having <200 CD4 were the same in Spaniards and migrants, but the association was stronger in the latter group.13
The objectives of this paper are to present data on the immunological situation of the new HIV diagnoses reported to the SINIVIH in the period 2007–2011, and to determine whether predictors for having <200 CD4 at entry to care are different from predictors of having <350 CD4.
MethodsAll people newly diagnosed with HIV – according to the European case definition14 – and reported during the period 2007–2011 to the SINIVIH in the AR of Asturias, Balearic Islands, Canary Islands, Catalonia, Ceuta, Extremadura, Galicia, Madrid, Navarre, Basque Country and La Rioja, were included in the study. These regions comprise 54% of the total Spanish population and were selected for the analysis because they reported to the SINIVIH during the whole study period.
Information was collected, using the standard surveillance forms, on the following variables: date of HIV diagnosis, date of notification, age, gender, reporting autonomous region, transmission mode, first CD4 cell count performed after HIV diagnosis and country or subcontinent of origin. Data sources were the attending clinicians and the laboratories, and information was completed by reviewing clinical records where necessary.
The study was performed according to the Spanish legislation on data protection which does not require informed consent for the collection of surveillance data, as is the case in this study (Ley 33/2011, de 4 de octubre, General de Salud Pública, artículo 41.2).
Elimination of duplicate reports was made in each AR by public health staff, but inter-regional duplicates were not eliminated since data were sent without personal identifiers to the National Center of Epidemiology, where the analysis was performed.
For the purpose of this study, “advanced HIV disease” (AD) was defined as the presence of a CD4 count below 200cells/μL in the first analysis performed after HIV diagnosis. “Late presentation” (LP) was defined as the presence of a CD4 count below 350cells/μL in the first analysis performed after HIV diagnosis.
Data distributions by variables related to person, place and time were performed. The χ2 test was used to evaluate the association between categorical variables. The odds ratio (OR) and its 95% confidence interval (95%CI) were used as the measure of association. To identify variables associated with AD or LP, multivariable logistic regression models were fit in the overall population and disaggregated by sex; for these models only cases with information on CD4 were used. Additionally, in order to assess the potential bias introduced by excluding sicker patients without a CD4 count, a sensitivity analysis was performed, assuming that all patients without a CD4 count were late presenters. All statistical analyses were performed using Stata Statistical Software: Release 11.1, College Station, TX: Stata Corporation (2009).
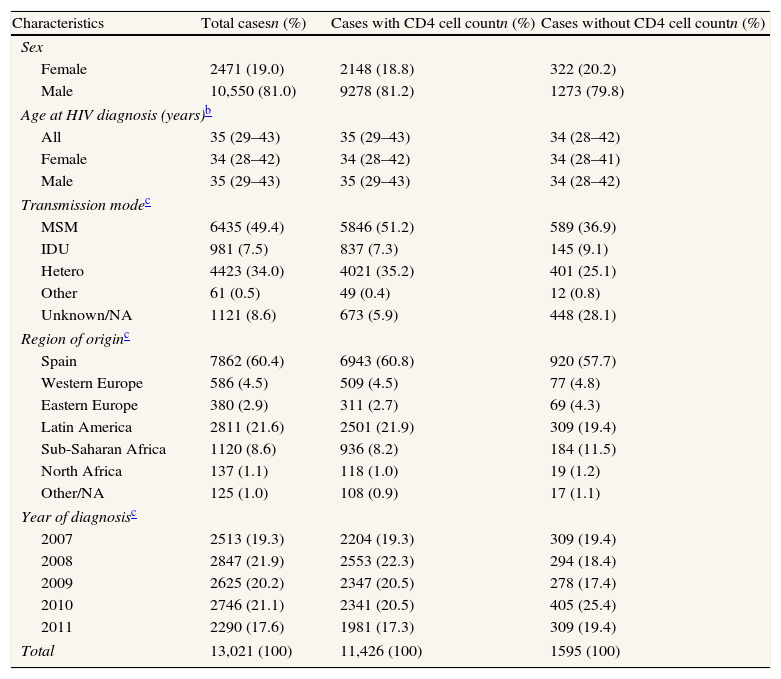
ResultsA total of 13,021 new HIV cases were reported during the study period, of which 11,426 (87.7%) had information on CD4 cell count at presentation. The majority (81%) of the new HIV diagnoses were male, and 83.4% had been infected through sexual contact, especially through sex between men; the median age at diagnosis was 35 years (inter-quartile range: 29–43). Cases with information on CD4 count differed from those without this information with regard to transmission mode, region of origin and year or diagnosis: Sub-Saharan migrants and injecting drug users (IDU) were over-represented among people lacking this information; furthermore, the proportion of cases with unknown information on transmission mode was higher among those without data on CD4 count (Table 1).
Characteristics of new HIV diagnoses. Spain,a 2007–2011.
| Characteristics | Total casesn (%) | Cases with CD4 cell countn (%) | Cases without CD4 cell countn (%) |
| Sex | |||
| Female | 2471 (19.0) | 2148 (18.8) | 322 (20.2) |
| Male | 10,550 (81.0) | 9278 (81.2) | 1273 (79.8) |
| Age at HIV diagnosis (years)b | |||
| All | 35 (29–43) | 35 (29–43) | 34 (28–42) |
| Female | 34 (28–42) | 34 (28–42) | 34 (28–41) |
| Male | 35 (29–43) | 35 (29–43) | 34 (28–42) |
| Transmission modec | |||
| MSM | 6435 (49.4) | 5846 (51.2) | 589 (36.9) |
| IDU | 981 (7.5) | 837 (7.3) | 145 (9.1) |
| Hetero | 4423 (34.0) | 4021 (35.2) | 401 (25.1) |
| Other | 61 (0.5) | 49 (0.4) | 12 (0.8) |
| Unknown/NA | 1121 (8.6) | 673 (5.9) | 448 (28.1) |
| Region of originc | |||
| Spain | 7862 (60.4) | 6943 (60.8) | 920 (57.7) |
| Western Europe | 586 (4.5) | 509 (4.5) | 77 (4.8) |
| Eastern Europe | 380 (2.9) | 311 (2.7) | 69 (4.3) |
| Latin America | 2811 (21.6) | 2501 (21.9) | 309 (19.4) |
| Sub-Saharan Africa | 1120 (8.6) | 936 (8.2) | 184 (11.5) |
| North Africa | 137 (1.1) | 118 (1.0) | 19 (1.2) |
| Other/NA | 125 (1.0) | 108 (0.9) | 17 (1.1) |
| Year of diagnosisc | |||
| 2007 | 2513 (19.3) | 2204 (19.3) | 309 (19.4) |
| 2008 | 2847 (21.9) | 2553 (22.3) | 294 (18.4) |
| 2009 | 2625 (20.2) | 2347 (20.5) | 278 (17.4) |
| 2010 | 2746 (21.1) | 2341 (20.5) | 405 (25.4) |
| 2011 | 2290 (17.6) | 1981 (17.3) | 309 (19.4) |
| Total | 13,021 (100) | 11,426 (100) | 1595 (100) |
HIV: human immunodeficiency virus; MSM: men who have sex with men; IDU: injecting drug use; hetero: heterosexual contact; NA: not available.
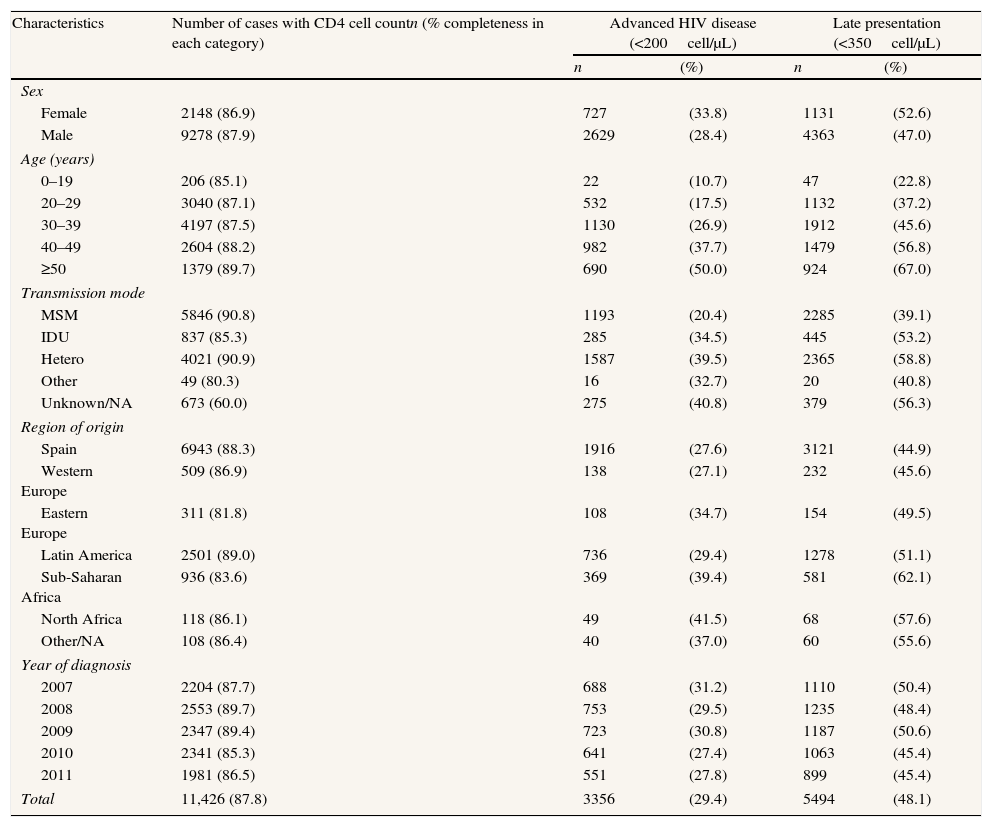
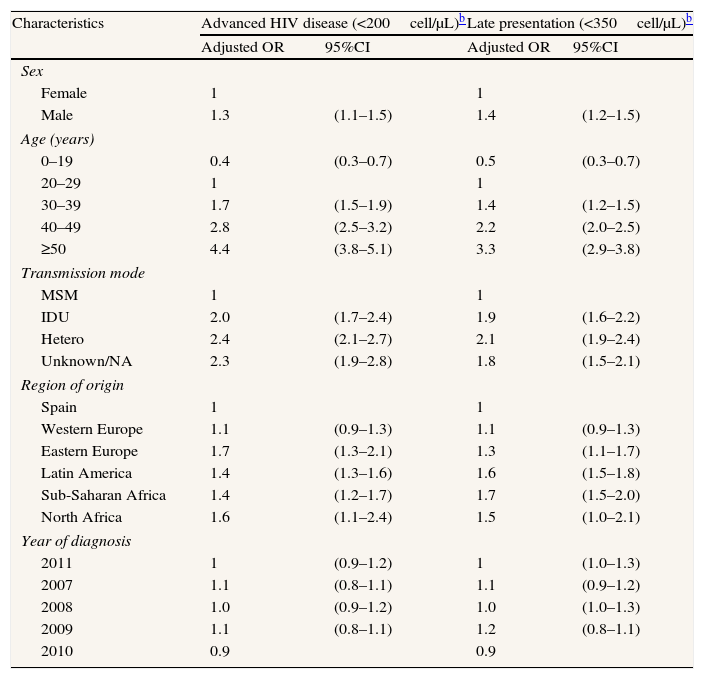
Median CD4 at presentation was 363 (inter-quartile range: 161–565). Overall, 3356 (29.4%) patients met the definition of AD. The proportion of patients with AD was higher in women, increased with age, and was highest among cases whose transmission mode was IDU or heterosexual as compared with men who have sex with men (MSM). By region of origin, AD prevalence was generally greater in migrants than in Spaniards (Table 2). In the multivariate analysis of the global data the probability of having AD was higher in men (OR: 1.3; 95%CI: 1.1–1.5), in the age groups 30–39 years (OR: 1.7; 95%CI: 1.5–1.9), 40–49 years (OR: 2.8; 95%CI: 2.5–3.2) and ≥50 years (OR: 4.4; 95%CI: 3.8–5.1), as compared to the age group 20–29 years; in people originating from East Europe (OR: 1.7; 95%CI: 1.3–2.1), Latin America (OR: 1.4; 95%CI: 1.3–1.6), Sub-Saharan Africa (OR: 1.4; 95%CI: 1.2–1.7), and North Africa (OR: 1.6; 95%CI: 1.1–2.4); and among IDU (OR: 2.0; 95%CI: 1.7–2.4) and those infected by heterosexual contact (OR: 2.4; 95%CI: 2.1–2.7) (Table 3).
Advanced HIV disease and late presentation in new HIV diagnoses. Spain,a 2007–2011.
| Characteristics | Number of cases with CD4 cell countn (% completeness in each category) | Advanced HIV disease (<200cell/μL) | Late presentation (<350cell/μL) | ||
| n | (%) | n | (%) | ||
| Sex | |||||
| Female | 2148 (86.9) | 727 | (33.8) | 1131 | (52.6) |
| Male | 9278 (87.9) | 2629 | (28.4) | 4363 | (47.0) |
| Age (years) | |||||
| 0–19 | 206 (85.1) | 22 | (10.7) | 47 | (22.8) |
| 20–29 | 3040 (87.1) | 532 | (17.5) | 1132 | (37.2) |
| 30–39 | 4197 (87.5) | 1130 | (26.9) | 1912 | (45.6) |
| 40–49 | 2604 (88.2) | 982 | (37.7) | 1479 | (56.8) |
| ≥50 | 1379 (89.7) | 690 | (50.0) | 924 | (67.0) |
| Transmission mode | |||||
| MSM | 5846 (90.8) | 1193 | (20.4) | 2285 | (39.1) |
| IDU | 837 (85.3) | 285 | (34.5) | 445 | (53.2) |
| Hetero | 4021 (90.9) | 1587 | (39.5) | 2365 | (58.8) |
| Other | 49 (80.3) | 16 | (32.7) | 20 | (40.8) |
| Unknown/NA | 673 (60.0) | 275 | (40.8) | 379 | (56.3) |
| Region of origin | |||||
| Spain | 6943 (88.3) | 1916 | (27.6) | 3121 | (44.9) |
| Western Europe | 509 (86.9) | 138 | (27.1) | 232 | (45.6) |
| Eastern Europe | 311 (81.8) | 108 | (34.7) | 154 | (49.5) |
| Latin America | 2501 (89.0) | 736 | (29.4) | 1278 | (51.1) |
| Sub-Saharan Africa | 936 (83.6) | 369 | (39.4) | 581 | (62.1) |
| North Africa | 118 (86.1) | 49 | (41.5) | 68 | (57.6) |
| Other/NA | 108 (86.4) | 40 | (37.0) | 60 | (55.6) |
| Year of diagnosis | |||||
| 2007 | 2204 (87.7) | 688 | (31.2) | 1110 | (50.4) |
| 2008 | 2553 (89.7) | 753 | (29.5) | 1235 | (48.4) |
| 2009 | 2347 (89.4) | 723 | (30.8) | 1187 | (50.6) |
| 2010 | 2341 (85.3) | 641 | (27.4) | 1063 | (45.4) |
| 2011 | 1981 (86.5) | 551 | (27.8) | 899 | (45.4) |
| Total | 11,426 (87.8) | 3356 | (29.4) | 5494 | (48.1) |
HIV: Human immunodeficiency virus; MSM: men who have sex with men; IDU: injecting drug use; hetero: heterosexual contact; NA: not available.
Factors associated with advanced disease and late presentation in newly diagnosed HIV infections. Spain,a 2007–2011. N=11,426.
| Characteristics | Advanced HIV disease (<200cell/μL)b | Late presentation (<350cell/μL)b | ||
| Adjusted OR | 95%CI | Adjusted OR | 95%CI | |
| Sex | ||||
| Female | 1 | 1 | ||
| Male | 1.3 | (1.1–1.5) | 1.4 | (1.2–1.5) |
| Age (years) | ||||
| 0–19 | 0.4 | (0.3–0.7) | 0.5 | (0.3–0.7) |
| 20–29 | 1 | 1 | ||
| 30–39 | 1.7 | (1.5–1.9) | 1.4 | (1.2–1.5) |
| 40–49 | 2.8 | (2.5–3.2) | 2.2 | (2.0–2.5) |
| ≥50 | 4.4 | (3.8–5.1) | 3.3 | (2.9–3.8) |
| Transmission mode | ||||
| MSM | 1 | 1 | ||
| IDU | 2.0 | (1.7–2.4) | 1.9 | (1.6–2.2) |
| Hetero | 2.4 | (2.1–2.7) | 2.1 | (1.9–2.4) |
| Unknown/NA | 2.3 | (1.9–2.8) | 1.8 | (1.5–2.1) |
| Region of origin | ||||
| Spain | 1 | 1 | ||
| Western Europe | 1.1 | (0.9–1.3) | 1.1 | (0.9–1.3) |
| Eastern Europe | 1.7 | (1.3–2.1) | 1.3 | (1.1–1.7) |
| Latin America | 1.4 | (1.3–1.6) | 1.6 | (1.5–1.8) |
| Sub-Saharan Africa | 1.4 | (1.2–1.7) | 1.7 | (1.5–2.0) |
| North Africa | 1.6 | (1.1–2.4) | 1.5 | (1.0–2.1) |
| Year of diagnosis | ||||
| 2011 | 1 | (0.9–1.2) | 1 | (1.0–1.3) |
| 2007 | 1.1 | (0.8–1.1) | 1.1 | (0.9–1.2) |
| 2008 | 1.0 | (0.9–1.2) | 1.0 | (1.0–1.3) |
| 2009 | 1.1 | (0.8–1.1) | 1.2 | (0.8–1.1) |
| 2010 | 0.9 | 0.9 | ||
VIH: Human immunodeficiency virus; OR: odds ratio; CI: confidence interval; MSM: men who have sex with men; IDU: injecting drug use; hetero: heterosexual contact; NA: not available.
A total of 5494 (48.1%) subjects presented late. The groups most affected by LP were the same as those affected by AD and the associations were in the same direction; nevertheless in the multivariate analysis there were slight differences in the strength of the associations (Tables 2 and 3).
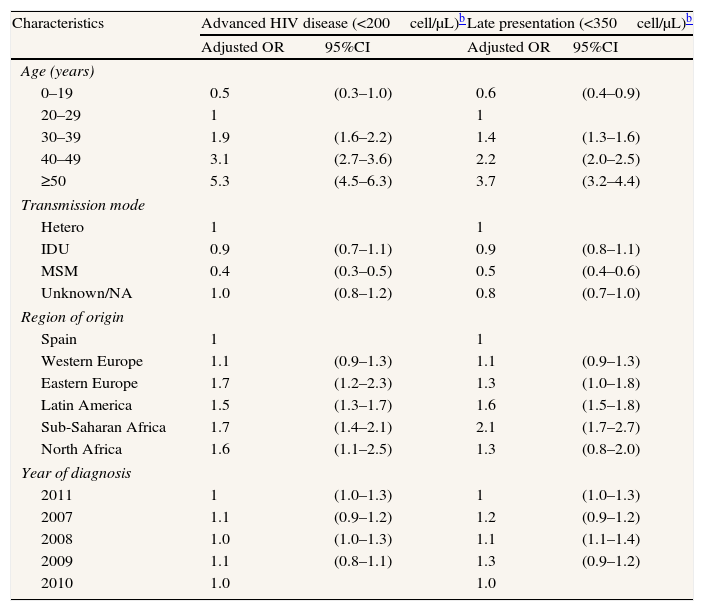
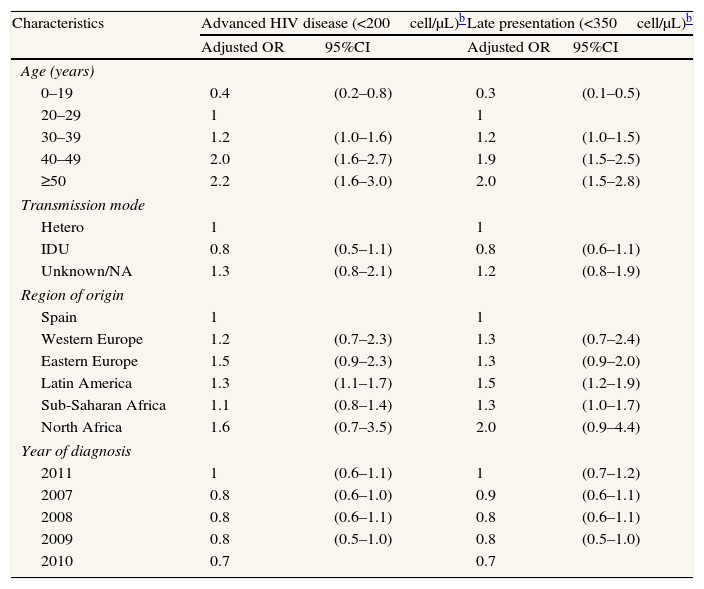
When disaggregating by sex, the multivariate analyses show different effect of age and region of origin in men than in women. In men, AD and LP clearly increase for cases older than 29 while among women the effect is weaker and the increase in the age group 30–39 is on the edge of statistical significance. The effect of region of origin is also weaker in women than in men (Tables 4 and 5).
Factors associated with advanced disease and late presentation in newly diagnosed HIV infections in men. Spain,a 2007–2011. N=9278.
| Characteristics | Advanced HIV disease (<200cell/μL)b | Late presentation (<350cell/μL)b | ||
| Adjusted OR | 95%CI | Adjusted OR | 95%CI | |
| Age (years) | ||||
| 0–19 | 0.5 | (0.3–1.0) | 0.6 | (0.4–0.9) |
| 20–29 | 1 | 1 | ||
| 30–39 | 1.9 | (1.6–2.2) | 1.4 | (1.3–1.6) |
| 40–49 | 3.1 | (2.7–3.6) | 2.2 | (2.0–2.5) |
| ≥50 | 5.3 | (4.5–6.3) | 3.7 | (3.2–4.4) |
| Transmission mode | ||||
| Hetero | 1 | 1 | ||
| IDU | 0.9 | (0.7–1.1) | 0.9 | (0.8–1.1) |
| MSM | 0.4 | (0.3–0.5) | 0.5 | (0.4–0.6) |
| Unknown/NA | 1.0 | (0.8–1.2) | 0.8 | (0.7–1.0) |
| Region of origin | ||||
| Spain | 1 | 1 | ||
| Western Europe | 1.1 | (0.9–1.3) | 1.1 | (0.9–1.3) |
| Eastern Europe | 1.7 | (1.2–2.3) | 1.3 | (1.0–1.8) |
| Latin America | 1.5 | (1.3–1.7) | 1.6 | (1.5–1.8) |
| Sub-Saharan Africa | 1.7 | (1.4–2.1) | 2.1 | (1.7–2.7) |
| North Africa | 1.6 | (1.1–2.5) | 1.3 | (0.8–2.0) |
| Year of diagnosis | ||||
| 2011 | 1 | (1.0–1.3) | 1 | (1.0–1.3) |
| 2007 | 1.1 | (0.9–1.2) | 1.2 | (0.9–1.2) |
| 2008 | 1.0 | (1.0–1.3) | 1.1 | (1.1–1.4) |
| 2009 | 1.1 | (0.8–1.1) | 1.3 | (0.9–1.2) |
| 2010 | 1.0 | 1.0 | ||
VIH: Human immunodeficiency virus; OR: odds ratio; CI: confidence interval; MSM: men who have sex with men; IDU: injecting drug use; hetero: heterosexual contact; NA: not available.
Factors associated with advanced disease and late presentation in newly diagnosed HIV infections in women. Spain,a 2007–2011. N=2148.
| Characteristics | Advanced HIV disease (<200cell/μL)b | Late presentation (<350cell/μL)b | ||
| Adjusted OR | 95%CI | Adjusted OR | 95%CI | |
| Age (years) | ||||
| 0–19 | 0.4 | (0.2–0.8) | 0.3 | (0.1–0.5) |
| 20–29 | 1 | 1 | ||
| 30–39 | 1.2 | (1.0–1.6) | 1.2 | (1.0–1.5) |
| 40–49 | 2.0 | (1.6–2.7) | 1.9 | (1.5–2.5) |
| ≥50 | 2.2 | (1.6–3.0) | 2.0 | (1.5–2.8) |
| Transmission mode | ||||
| Hetero | 1 | 1 | ||
| IDU | 0.8 | (0.5–1.1) | 0.8 | (0.6–1.1) |
| Unknown/NA | 1.3 | (0.8–2.1) | 1.2 | (0.8–1.9) |
| Region of origin | ||||
| Spain | 1 | 1 | ||
| Western Europe | 1.2 | (0.7–2.3) | 1.3 | (0.7–2.4) |
| Eastern Europe | 1.5 | (0.9–2.3) | 1.3 | (0.9–2.0) |
| Latin America | 1.3 | (1.1–1.7) | 1.5 | (1.2–1.9) |
| Sub-Saharan Africa | 1.1 | (0.8–1.4) | 1.3 | (1.0–1.7) |
| North Africa | 1.6 | (0.7–3.5) | 2.0 | (0.9–4.4) |
| Year of diagnosis | ||||
| 2011 | 1 | (0.6–1.1) | 1 | (0.7–1.2) |
| 2007 | 0.8 | (0.6–1.0) | 0.9 | (0.6–1.1) |
| 2008 | 0.8 | (0.6–1.1) | 0.8 | (0.6–1.1) |
| 2009 | 0.8 | (0.5–1.0) | 0.8 | (0.5–1.0) |
| 2010 | 0.7 | 0.7 | ||
VIH: Human immunodeficiency virus; OR: odds ratio; CI: confidence interval; IDU: injecting drug use; hetero: heterosexual contact; NA: not available.
When the sensitivity analysis assuming that cases without information on CD4 were late presenters was performed factors associated with AD and LP did not change.
DiscussionThis study presents surveillance data on CD4 count at presentation for care in new HIV diagnoses in a setting covering 54% of the total Spanish population. The results show that subjects who had information on CD4 cell count are different from those who did not, that almost half of new HIV diagnoses presented late to medical care and 30% had AD, and that predictors of LP and AD are the same.
Surveillance data on CD4 count at entry into care are scarce in the EU.12 In this study information was available in almost 90% of registries; however, the results show that the lack of information is not randomly distributed, suggesting that traditionally disadvantaged subjects, such as IDU and migrants, might have problems in accessing care after being diagnosed, even if they are entitled to free heath care, as is the case in Spain.
The prevalence of AD and LP is similar to that found in France or the UK, and higher than the figures reported in The Netherlands or the Czech Republic.12 Outside the EU, studies carried out – using the same definition – in the United States and Hong Kong report a greater proportion of AD.15,16
In Spain, HIV testing is part of routine prenatal care, and this probably explains, at least to some extent, why women are less likely to present late. The fact that (as shown in the multivariate analyses disaggregated by sex) the effect of age and region of origin on AD and LP are weaker in women than in men also support this explanation. This has also been observed in other developed countries where antenatal HIV screening is the rule.17 Data from the United Kingdom show that median CD4 at HIV diagnosis is significantly higher in pregnant women than in women diagnosed in other units and in heterosexual men.18
The association of AD with age is already known.13,15,16,19–25 Age is also associated with LP. The most widely accepted explanation is that risk perception and frequency of testing decrease with increasing age.20,25 Another possible explanation relates to the natural history of the disease, which clinically and immunologically evolves more rapidly in older patients.25
In comparison with other transmission modes, MSM are the least likely to present late, while people infected through heterosexual contact are more likely, findings also common to other countries.16,21,23,24 These results probably reflect risk perceptions on the part of both patients and providers, and point to the need to scale up HIV testing among heterosexuals, in particular among men.
Spain experienced overlapping epidemics of HIV and heroin injection from the 1980s to the mid-1990s.26 The high likelihood of AD/LP in IDU is surprising, since risk perception in this group is very high and HIV testing is routinely offered to patients in drug treatment centers, prisons and at all contacts with the health care system. A possible explanation could be that rather than being the consequence of late diagnosis this finding is the result of worse linkage to care among IDU due to barriers in accessing care specific to this group. The fact that in our data, the proportion of IDU with a CD4 count performed within the first 3 months after HIV diagnosis is lower than for other transmission modes (60% vs. 77%, p<0.05) supports this explanation.
Previously published studies, both in Spain and abroad, identify being a migrant as a risk factor for late HIV diagnosis and presentation for care.13,19,27,28 Worries about discrimination, language barriers, the perception of HIV as a deadly disease, and lack of confidence about the confidentiality of results have been noted as barriers for HIV testing among migrants,29,30 and migrants, in particular illegal migrants, have restricted access to care in most countries.31 Some of these factors might be operating in Spain, even though all migrants are entitled to HIV care on the same basis as Spaniards. On the other hand, immigration is a very recent phenomenon in Spain and it is possible that, to some extent, figures for AD and LP in immigrants reflect factors operating in their country of origin rather than in Spain. Unfortunately, date of entry into Spain is not yet collected in the SINIVIH; thus this hypothesis cannot be explored further.
This study has some limitations. The results cannot be extrapolated to the whole country because data are not countrywide. Nevertheless, study coverage is high, under-reporting is estimated to be moderate, and participating AR do not differ from the rest in terms of factors influencing AD/LP, since HIV care is provided free of charge, in the context of a universal health care system, in the whole country. Furthermore, due to the lack of information on the concomitant diagnosis of AIDS and HIV, it could be argued that our data underestimate AD and LP, since some authors include presence of AIDS as an additional defining criteria for the two events.7 While it is true that this information is not currently available in all participating AR, some do collect it; analysis of available data show that if this criterion were included in the definition of AD and LP, the proportion of AD in our data would increase from 29.4% to 32.7%, and the proportion of LP from 48.1% to 50.0%. On the other hand, since the SINIVIH has been implemented only recently in some of the participating AR, it is possible that some cases reported as new HIV diagnoses in the study period were actually previously diagnosed patients falsely classified as new due to the lack of an established HIV case registry; if this were the case, both AD and LP may be overestimated in this study. Finally, duplicates within each autonomous region have been eliminated but this is not the case with those within autonomous regions. We think that between 5–10% of registries could be duplicated, in which case the incidence of new HIV diagnoses would be overestimated; however, it is not clear how this would affect late presentation and factors associated with it. Nevertheless we expect to be able to eliminate duplicates within autonomous regions in the near future.
In summary, this study shows that one out of two new HIV diagnoses in Spain presents late for care, and that some sub-populations are more vulnerable than others to this problem. Recently, guidelines for scaling-up HIV testing have been published by some European countries and the ECDC.32 In Spain, a working group aiming to accomplish a similar task, including appropriate linkage to HIV care, has been appointed. Hopefully, implementation of guidelines in the near future will help to decrease late access to HIV diagnosis and care. Finally, enhanced surveillance, coupled with implementation of SINIVIH in the whole country, is essential to monitor guidelines implementation and provide better information on LP and its determinants at both national and international levels.
Advanced disease and late presentation remains a major challenge for the control of HIV. Reducing time between infection and entry to care is a priority. In Spain, epidemiological surveillance on new HIV diagnosis is performed through the Sistema de Información sobre Nuevos Diagnósticos de VIH which collects epidemiological and clinical data on people newly diagnosed.
What does this study add to the literature?In Spain, data on CD4 count at entry into care were available in almost 90% of new HIV diagnoses notified to the surveillance system in the period 2007–2011. Some socially disadvantaged groups, such as IDU and migrants, were more likely to have missing data on CD4 count, suggesting that they might have problems in accessing care after being diagnosed. In the study one out of two new HIV diagnoses in Spain presented late for care. Male patients, migrants and people infected through heterosexual contact or sharing of injection equipment were more likely to present late for care.
Pere Godoy, M.D.
Authorship contributionsThe number of authors is justified by the existence of a person responsible for the new HIV diagnoses system in each autonomous region. All authors participated in data collection and aggregation and quality control of the databases in their corresponding autonomous regions and also performed in-depth reviews of all the drafts of the manuscript. J. Oliva performed data aggregation, quality control, planning and analysis of the overall database, prepared the tables, wrote the first draft of the manuscript and participated in successive drafts up to the final version. M. Diez conceived the study and participated in all drafts up to the final version. S. Galindo contributed to data management and analysis of the overall database and performed in-depth revisions of all the drafts of the manuscript. C. Cevallos, A. Izquierdo, J. Cereijo, A. Arrillaga, A. Nicolau, A. Fernández, M. Álvarez, J. Castilla, E. Martínez, I. López and N. Vives carried out data collection and aggregation and quality control in the databases of their autonomous regions and performed in-depth revisions of all the drafts of the manuscript. All authors have reviewed and approved the final manuscript.
FundingNone.
Conflicts of interestNone.
The authors wish to thank all the health professionals that reported cases and contributed in one way or another to improving the quality of the data. The authors also thank Kathy Fitch for her review of the English translation.