We present the protocol of a large population-based case-control study of 5 common tumors in Spain (MCC-Spain) that evaluates environmental exposures and genetic factors.
MethodsBetween 2008-2013, 10,183 persons aged 20-85 years were enrolled in 23 hospitals and primary care centres in 12 Spanish provinces including 1,115 cases of a new diagnosis of prostate cancer, 1,750 of breast cancer, 2,171 of colorectal cancer, 492 of gastro-oesophageal cancer, 554 cases of chronic lymphocytic leukaemia (CLL) and 4,101 population-based controls matched by frequency to cases by age, sex and region of residence. Participation rates ranged from 57% (stomach cancer) to 87% (CLL cases) and from 30% to 77% in controls. Participants completed a face-to-face computerized interview on sociodemographic factors, environmental exposures, occupation, medication, lifestyle, and personal and family medical history. In addition, participants completed a self-administered food-frequency questionnaire and telephone interviews. Blood samples were collected from 76% of participants while saliva samples were collected in CLL cases and participants refusing blood extractions. Clinical information was recorded for cases and paraffin blocks and/or fresh tumor samples are available in most collaborating hospitals. Genotyping was done through an exome array enriched with genetic markers in specific pathways. Multiple analyses are planned to assess the association of environmental, personal and genetic risk factors for each tumor and to identify pleiotropic effects.
DiscussionThis study, conducted within the Spanish Consortium for Biomedical Research in Epidemiology & Public Health (CIBERESP), is a unique initiative to evaluate etiological factors for common cancers and will promote cancer research and prevention in Spain.
Presentamos el protocolo del estudio caso-control de base poblacional de 5 tumores comunes en España (MCC-Spain) que evalúa factores ambientales y genéticos.
MétodosDurante 2008-2013, se reclutaron 10.183 sujetos entre 20-85 años en 23 hospitales de 12 provincias españolas, incluyendo 1.115 casos de cáncer de próstata, 1.750 de mama, 2.171 colorrectal, 492 gastro-esofágicos, 554 de leucemia linfática crónica (LLC) y 4.101 controles poblacionales emparejados por frecuencia por edad, sexo y región de residencia. Las tasas de participación varían del 57% (cáncer de estómago) al 87% (casos de LLC) y del 30% al 77% en controles. Los participantes respondieron una entrevista personal informatizada sobre factores socio-demográficos, exposiciones ambientales, ocupación, medicación, estilos de vida, e historia médica personal y familiar. Además, cumplimentaron un cuestionario alimentario y realizaron entrevistas telefónicas. Se recogió sangre del 76% de los participantes y saliva para los casos de LLC y participantes que rechazaron la donación de sangre. En los casos, se recogió información clínica y se dispone de muestras de tumor fresco o parafinado a través de los biobancos de los hospitales. Se realizó el genotipado con un array de exoma suplementado con marcadores en pathways específicos. Se han planificado diversos análisis para evaluar la asociación de factores genéticos, personales y ambientales para cada tumor e identificar efectos pleiotrópicos.
DiscusiónEste estudio, desarrollado en el Consorcio de Investigación Biomédica de Epidemiología y Salud Pública (CIBERESP), es una iniciativa única para evaluar factores etiológicos de tumores comunes y promoverá la investigación en cáncer y prevención en España.
A population-based multicase-control study (MCC-Spain) was launched in 2007 by the Consortium for Biomedical Research in Epidemiology & Public Health (CIBERESP) to evaluate the influence of environmental exposures and their interaction with genetic factors in three of the most common tumours in Spain (prostate, female breast, colorectal),1 in which etiological causes remain largely unknown. Gastric cancer was also included due to its large geographical variation within Spain,2 which points to the not well identified persistent environmental exposure in high risk areas (i.e. Castilla y León). A fifth neoplasm, chronic lymphocytic leukaemia (CLL) was included later, as a result of the collaboration with the International Cancer Genome Consortium (ICGC).
Diet, physical activity, obesity and family history are common risk factors for the tumours examined3,4. Moreover, environmental and occupational factors have also been investigated in relation to these tumours,5–7 but not in the Spanish population. Regarding infectious hypothesis, the best established risk factor for stomach cancer is infection with Helicobacter pylori (H. pylori), while infectious mononucleosis and high levels of Epstein-Barr Virus (EBV) antibodies have been associated with CLL.8 Non-steroidal anti-inflammatory drugs (NSAIDs) and statins may be protective for several of the included tumours. Genome Wide Association Studies (GWAS) have identified numerous low penetrance variants for colorectal, breast, and prostate cancer and CLL. 9–12 However, in spite of all the research conducted so far, the causes of this group of tumours are not well understood.
The aim of this study is to assess the association between environmental exposures and individual factors, including genetic susceptibility, and the occurrence of these cancers in Spain. In summary, MCC-Spain intends to explore and combine different approaches in order to identify new risk factors of to provide new data that might help to prevent their occurrence in the future. The specific objectives of the study are related to: a) environmental exposures, including drinking water contaminants, heavy metals and endocrine disruptors exposure; b) socioeconomic factors and occupational exposures, including disruption of the circadian rhythm through shift work; c) lifestyle factors, smoking, nutrition, obesity and physical activity; d) medical history and consumption of specific drugs; e) hormonal factors, including exposures in early stages of life; f) infectious agents; and g) family history of cancer and genetic variation. Main effects and interactions, specifically with genetic factors, will be analyzed together with an evaluation by tumour subphenotypes.
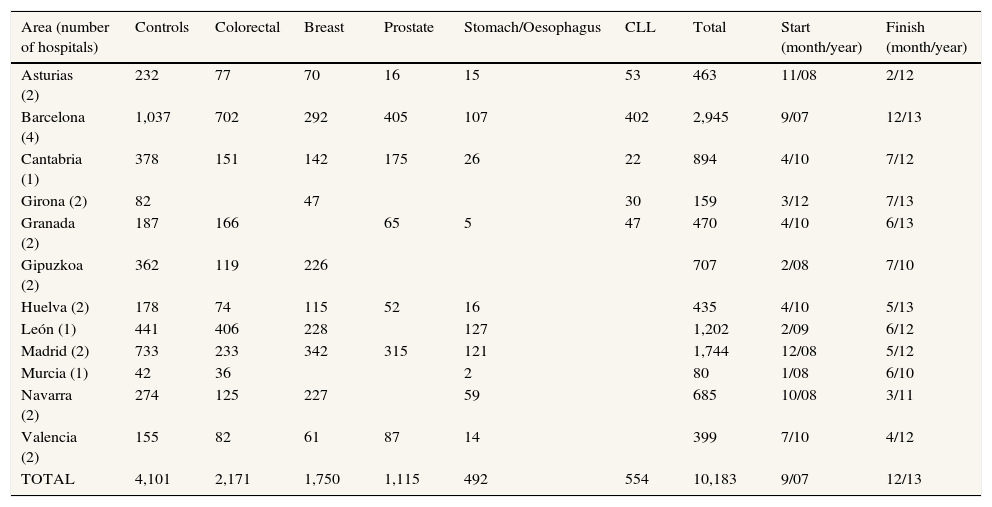
METHODSStudy designMCC-Spain is a population-based multicase-control study carried out between September 2008 and December 2013 in 12 Spanish provinces (Asturias, Barcelona, Cantabria, Girona, Granada, Gipuzkoa, Huelva, León, Madrid, Murcia, Navarra and Valencia). Recruitment of cases and controls was performed simultaneously: study personnel contacted newly diagnosed cancer cases in the 23 collaborating hospitals, and invited through the telephone population controls, who had been randomly selected from the administrative records of selected primary care health centres located within these hospitals’ catchment area. In total, the study recruited 10,183 subjects (Table 1). All participants had to be 20-85 years, to have resided in the catchment area for at least 6 months prior to recruitment, and to be able to answer the epidemiological questionnaire. Each province recruited cases of at least two different tumour sites. Cases were identified, as soon as possible after the diagnosis was made, through active search that included periodical visits to the collaborating hospital departments (i.e. gynaecology, urology, gastroenterology, oncology, general surgery, radiotherapy, and pathology departments). We included histologically confirmed incident cases of cancer of the prostate (International Classification of Diseases 10th Revision [ICD-10]: C61, D07.5), breast (C50, D05.1, D05.7), colon or rectum (C18, C19, C20, D01.0, D01.1, D01.2), stomach (C16, D00.2), lower third of the oesophagus (C15.5), or chronic lymphocytic leukaemia and small lymphocytic lymphoma (C91.1), with no prior history of the disease, and diagnosed within the recruitment period, which differed by province; in CLL prevalent cases were also recruited. Controls were frequency-matched to cases, by age, sex and region, ensuring that in each region there was at least one control of the same sex and 5-year interval for each case. For each control needed, a total of five potential participants of similar age, sex and hospital catchment area were randomly selected from the general practitioner lists. If contact with the first person of this list was not possible after a minimum of five tries at different times of the day, or if he/she refused to participate, the following person of the list was approached.
Number of cases and controls with complete interviews by tumour type and geographic area.
| Area (number of hospitals) | Controls | Colorectal | Breast | Prostate | Stomach/Oesophagus | CLL | Total | Start (month/year) | Finish (month/year) |
|---|---|---|---|---|---|---|---|---|---|
| Asturias (2) | 232 | 77 | 70 | 16 | 15 | 53 | 463 | 11/08 | 2/12 |
| Barcelona (4) | 1,037 | 702 | 292 | 405 | 107 | 402 | 2,945 | 9/07 | 12/13 |
| Cantabria (1) | 378 | 151 | 142 | 175 | 26 | 22 | 894 | 4/10 | 7/12 |
| Girona (2) | 82 | 47 | 30 | 159 | 3/12 | 7/13 | |||
| Granada (2) | 187 | 166 | 65 | 5 | 47 | 470 | 4/10 | 6/13 | |
| Gipuzkoa (2) | 362 | 119 | 226 | 707 | 2/08 | 7/10 | |||
| Huelva (2) | 178 | 74 | 115 | 52 | 16 | 435 | 4/10 | 5/13 | |
| León (1) | 441 | 406 | 228 | 127 | 1,202 | 2/09 | 6/12 | ||
| Madrid (2) | 733 | 233 | 342 | 315 | 121 | 1,744 | 12/08 | 5/12 | |
| Murcia (1) | 42 | 36 | 2 | 80 | 1/08 | 6/10 | |||
| Navarra (2) | 274 | 125 | 227 | 59 | 685 | 10/08 | 3/11 | ||
| Valencia (2) | 155 | 82 | 61 | 87 | 14 | 399 | 7/10 | 4/12 | |
| TOTAL | 4,101 | 2,171 | 1,750 | 1,115 | 492 | 554 | 10,183 | 9/07 | 12/13 |
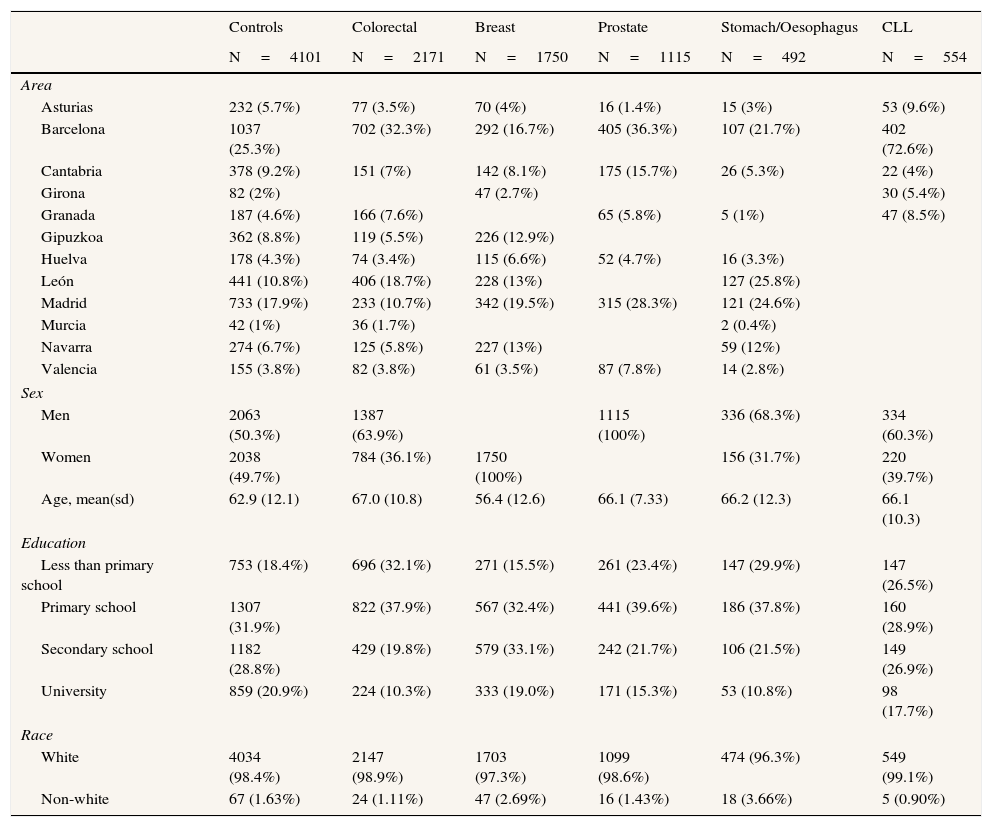
In Table 2 we present the main characteristics of the study population.
Main characteristics of the population of the MCC-Spain study.
| Controls | Colorectal | Breast | Prostate | Stomach/Oesophagus | CLL | |
|---|---|---|---|---|---|---|
| N=4101 | N=2171 | N=1750 | N=1115 | N=492 | N=554 | |
| Area | ||||||
| Asturias | 232 (5.7%) | 77 (3.5%) | 70 (4%) | 16 (1.4%) | 15 (3%) | 53 (9.6%) |
| Barcelona | 1037 (25.3%) | 702 (32.3%) | 292 (16.7%) | 405 (36.3%) | 107 (21.7%) | 402 (72.6%) |
| Cantabria | 378 (9.2%) | 151 (7%) | 142 (8.1%) | 175 (15.7%) | 26 (5.3%) | 22 (4%) |
| Girona | 82 (2%) | 47 (2.7%) | 30 (5.4%) | |||
| Granada | 187 (4.6%) | 166 (7.6%) | 65 (5.8%) | 5 (1%) | 47 (8.5%) | |
| Gipuzkoa | 362 (8.8%) | 119 (5.5%) | 226 (12.9%) | |||
| Huelva | 178 (4.3%) | 74 (3.4%) | 115 (6.6%) | 52 (4.7%) | 16 (3.3%) | |
| León | 441 (10.8%) | 406 (18.7%) | 228 (13%) | 127 (25.8%) | ||
| Madrid | 733 (17.9%) | 233 (10.7%) | 342 (19.5%) | 315 (28.3%) | 121 (24.6%) | |
| Murcia | 42 (1%) | 36 (1.7%) | 2 (0.4%) | |||
| Navarra | 274 (6.7%) | 125 (5.8%) | 227 (13%) | 59 (12%) | ||
| Valencia | 155 (3.8%) | 82 (3.8%) | 61 (3.5%) | 87 (7.8%) | 14 (2.8%) | |
| Sex | ||||||
| Men | 2063 (50.3%) | 1387 (63.9%) | 1115 (100%) | 336 (68.3%) | 334 (60.3%) | |
| Women | 2038 (49.7%) | 784 (36.1%) | 1750 (100%) | 156 (31.7%) | 220 (39.7%) | |
| Age, mean(sd) | 62.9 (12.1) | 67.0 (10.8) | 56.4 (12.6) | 66.1 (7.33) | 66.2 (12.3) | 66.1 (10.3) |
| Education | ||||||
| Less than primary school | 753 (18.4%) | 696 (32.1%) | 271 (15.5%) | 261 (23.4%) | 147 (29.9%) | 147 (26.5%) |
| Primary school | 1307 (31.9%) | 822 (37.9%) | 567 (32.4%) | 441 (39.6%) | 186 (37.8%) | 160 (28.9%) |
| Secondary school | 1182 (28.8%) | 429 (19.8%) | 579 (33.1%) | 242 (21.7%) | 106 (21.5%) | 149 (26.9%) |
| University | 859 (20.9%) | 224 (10.3%) | 333 (19.0%) | 171 (15.3%) | 53 (10.8%) | 98 (17.7%) |
| Race | ||||||
| White | 4034 (98.4%) | 2147 (98.9%) | 1703 (97.3%) | 1099 (98.6%) | 474 (96.3%) | 549 (99.1%) |
| Non-white | 67 (1.63%) | 24 (1.11%) | 47 (2.69%) | 16 (1.43%) | 18 (3.66%) | 5 (0.90%) |
CLL: lymphocytic leukaemia.
Response rates were calculated using subjects interviewed in the numerator, and all subjects including refusals in the denominator.13,14 For cases, these response rates were 68% for colorectal cancer cases, 71% for breast, 72% for prostate, 57% for gastric and 87% for CLL. In controls, mean participation rate of controls was 53% and varied by region. For 22% of the subjects the phone contact was not possible due wrong phone number or no-answer.
Questionnaires, biological samples, hospital records and anthropometric measurementsA structured computerized epidemiological questionnaire was administered by trained personnel in a face-to-face interview (http://www.mccspain.org). The average duration of the interview was 70minutes (range 30-130). Information was collected on socio-demographic factors, residential history, lifelong retrospective environmental exposures, including water consumption and use (showering, bathing, swimming in pools), occupational history -including night shift-, medication, lifestyles–smoking, alcohol consumption, physical activity, use of cosmetic products-, sunbathing and sleeping habits, personal/family medical history and quality of the interview. Missing values on key variables and specific questions on additional study objectives (e.g. questions on disruption of the circadian rhythm) were completed through subsequent telephone contact. The main characteristics of the study population are presented in Table 2. After the interview, biological samples and anthropometric data were obtained following the study protocol. Height and weight at different ages were self-reported and waist and hip circumference were measured with a tape. Subjects were provided a semi-quantitative Food Frequency Questionnaire (FFQ), which was a modified version from a previously validated instrument in Spain15 to include regional products. It included 140 food items, and assessed usual dietary intake during the previous year. Portion sizes were specified for each item, and photographs were used to define degrees of doneness. Information on consumption of vitamin and mineral supplements and on important changes on dietary habits in the past 5 years were also collected. The FFQ was self-administered and returned by mail or filled out face to face (global response rate 88%).
When feasible, 27ml of peripheral blood was drawn from participants, which were aliquoted in whole blood, plasma, cellular fraction for DNA extraction, and serum and stored at -80°C. Saliva was collected for subjects refusing to donate blood and for all CLL cases, with the Oragene® DNA Kit and stored at room temperature until DNA extraction. We collected biological samples for DNA extraction for 96% of participants with interview (76% blood and 27% saliva) as well as toenail and hair samples were taken from participants (79% and 84% respectively). In 4 centres (Madrid, Cantabria, Asturias and Huelva, which include approximately 1/3 of the study participants) cases and controls also donated urine samples (60ml) that were aliquoted and frozen at -80°C. Fresh tumour biopsies or paraffin embedded samples are available in all participating hospitals.
Standardised basic clinical and pathological information on the diagnosis and treatment of tumours was collected from hospital records using a predefined format.
Sociodemographic, lifestyle and environmental factorsMCC-Spain will examine the Socioeconomic status will be examined using multilevel approaches that allow the evaluation of the role of structural socioeconomic factors on health. Environmental justice, proximity to green spaces and environmental pollution will be assessed through an evaluation of exposures proximate to the place of residence.
Lifestyle exposures are one of the main objectives of this study. Diet is examined through summary intakes of relevant food groups based on reported intake frequencies and portion size information. Food composition tables were developed and will be combined with reported intake frequencies and cooking method preferences to estimate intakes of nutrients, food contaminants (e.g. polycyclic aromatic hydrocarbons) and food properties (e.g. total antioxidant capacity. An a priori Mediterranean diet score, alternative diet patterns and a factor-analysis derived diet pattern will be examined. General and central obesity is examined together with leisure time physical activity and sedentary lifestyle. Numerous other potential risk factors that could be associated with the cancers investigated are examined. These include, among others, smoking habits, exposure to medical radiation, use of cosmetics, use of tight clothes and belts, exposure to sun or sleeping patterns.
Use of medical drugs was collected through personal interviews, mainly by indication. Information was coded (Anatomical Therapeutic Chemical-ATC code) to assess individual exposure to different drugs including statins and anti-inflammatory drugs, analgesics, hormones, antihypertensives, beta-blockers, bisphosphonates and corticosteroids.
Hormonal factors and endocrine disruption are also examined. Sex dimorphic phenotypes (finger ratio and anogenital distance) will be evaluated in relation to the development of breast and prostate cancer. The ratio of the length of the index finger and middle finger of both hands (2D: 4D) were measured using callipers with a resolution of 0.05mm,16 a validation study has shown a good repeatability of finger measurements.17 Anogenital distance was assessed in a subgroup of prostate cancer cases and controls.18 The influence of reproductive history, hormonal treatments (contraceptives, hormone replacement therapy) and influence of hormonal development (pattern of fat distribution at different ages, and height) will be examined. Endocrine disruptors (xenoestrogens and other persistent organic pollutants) will be measured in serum through a determination of the global xenoestrogenic burden (TEXB).19
Among environmental pollutants, evaluation of drinking water contaminants focuses on disinfection by products (such as trihalomethanes and haloacetic acids), nitrates and metals. Exposure data from water companies and municipalities, national surveillance data and new water analyses has been gathered.20,21 and modelled to estimate historical levels of pollutants in drinking water and combined with individual data from the questionnaire. Urinary trichloroacetic acid was measured in a subset of controls. MCC-Spain will also study environmental exposure to different metals, including Cd, Ni, Cr, As, Pb, Se, and Zn in relation to the five combining biomarker-based estimations with information based on the epidemiological information.
Occupational exposures will also be studied. All jobs conducted for more than one year were recorded with information regarding specific tasks, exposures and timing of the job. Jobs were coded by two experts following the Spanish National Classification of Occupations (CNO-94). The Spanish JEM, MatEmEsp, will be applied.22 Detailed information on work shift (rotating and night) and disruption of the circadian rhythm was also collected.
Other possible etiological factors have also been included in the project. Among them, several infections will be evaluated in relation to colorectal and gastric tumours and CLL. The role of H. pylori infection will be estimated using seroprevalence against several virulence antigens. In relation to CLL, seroprevalence of several polyomaviruses, herpesviruses, and Chlamydia trachomatis will be evaluated. Additionally the antibody response pattern to EBV will also be measured.
Extensive information on family history was collected to identify familial cases. This information will allow describing the typical family structure of study participants and, if genetic effects are identified, estimating their penetrance using Kin-cohort methods. In addition, genetic analyses will be carried out within MCC-Spain and also through participation in international consortia such as the prostate cancer consortium PRACTICAL (http://ccge.medschl.cam.ac.uk/consortia/practical/). The Infinium HumanExome BeadChip from Illumina was used to genotype >200,000 coding markers plus 6,000 additional custom variants on the pathways of interest such as inflammation, circadian rhythm or detoxification.
Ethics and availability of dataThe protocol of MCC-Spain was approved by the Ethics committees of the participating institutions. All participants were informed about the study objectives and signed an informed consent. Confidentiality of data is secured removing personal identifiers in the datasets. The database was registered in the Spanish Agency for Data Protection, number 2102672171. Permission to use the study database will be granted to researchers outside the study group, after revision and approval of each request by the Steering Committee. More details on the organization of the project can be found online at http://www.mccspain.org.
DISCUSSIONCancer locations and hypotheses examined in MCC-Spain were selected with a public health perspective to provide information useful for cancer prevention. MCC-Spain also aims to foster a network of research in cancer epidemiology in Spain.
The option of a single set of population controls and a single questionnaire for all tumours has the main benefit of the optimization of the economic resources. This approach has been successfully used previously. 23 On the other hand, the main drawback is the need to use the same tools to gather the information regarding risk factors for all types of cases.
The advantages and problems of the selection of population versus hospital controls have been extensively discussed.24,25 The evaluation of a variety of exposures makes hospital controls less suitable given the potential association between multiple diseases and exposures of interest. As expected from other population-based studies, participation rates of cases were higher than those of controls. Selecting controls through lists of general practitioners provides a representative sampling frame given the almost universal public coverage of the national health system in Spain. However, errors in these lists concerning personal data resulted in a lower than expected response rate.14
The study is population-based since we intended to recruit all cases with a first diagnosis of the studied tumors in the selected health areas, using for this purpose the reference hospital/hospitals in each area and identifying every new diagnosis of the studied cancers. We could not use population cancer registries to ascertain the number of cases lost since in most of the regions included in the study there was not any such registry, but we can certainly assume to be few. Potential misclassification of exposure is a major limitation of case-control studies. The implementation of a computerized questionnaire, training and continuous feedback to interviewers, and repeated interviews to complete missing values is likely to reduce errors.
A challenge of current cancer epidemiology research is to accurately define the molecular phenotype of tumours so that specific risk factors can be identified for each molecular subtype. All tumours have pathology slides in the reference hospitals that can be retrieved and some hospitals also have tumour banks that have collected fresh tumour tissue for some cases.
Finally, networking is among the major achievements of the study. MCC-Spain includes 17 different centres and has followed organizational procedures to promote the exchange of knowledge and experiences between centres.
Editor in chargeAlberto Ruano-Ravina.
Statement of authorshipAll authors have contributed to the conception and design of the study, and have acquired data, have been involved in drafting the manuscript. All authors read and approved the final manuscript.
FundingThe study was partially funded by the “Accion Transversal del Cancer”, approved on the Spanish Ministry Council on the 11th October 2007, by the Instituto de Salud Carlos III-FEDER (PI08/1770, PI08/0533, PI08/1359, PS09/00773, PS09/01286, PS09/01903, PS09/02078, PS09/01662, PI11/01403, PI11/01889, PI11/00226, PI11/01810, PI11/02213, PI12/00488, PI12/00265, PI12/01270, PI12/00715, PI12/00150), by the Fundación Marqués de Valdecilla (API 10/09), by the ICGC International Cancer Genome Consortium CLL, by the Junta de Castilla y León (LE22A10-2), by the Consejería de Salud of the Junta de Andalucía (PI-0571), by the Conselleria de Sanitat of the Generalitat Valenciana (AP_061/10), by the Recercaixa (2010ACUP 00310), by the Regional Government of the Basque Country by European Commission grants FOOD-CT-2006-036224-HIWATE, by the Spanish Association Against Cancer (AECC) Scientific Foundation, by the The Catalan Government DURSI grant 2009SGR1489.
Samples: Biological samples were stored at the Parc de Salut MAR Biobank (MARBiobanc; Barcelona) which is supported by Instituto de Salud Carlos III FEDER (RD09/0076/00036). Also at the Public Health Laboratory from Gipuzkoa and the Basque Biobank. Also sample collection was supported by the Xarxa de Bancs de Tumors de Catalunya sponsored by Pla Director d’Oncologia de Catalunya (XBTC). Biological samples were stored at the “Biobanco La Fe” which is supported by Instituto de Salud Carlos III (RD 09 0076/00021) and FISABIO biobanking, which is supported by Instituto de Salud Carlos III (RD09 0076/00058).
Genotyping: SNP genotyping services were provided by the Spanish “Centro Nacional de Genotipado” (CEGEN-ISCIII)" and by the Basque Biobank.
Conflict of interestThe authors declare that they have no conflicts of interest.
We thank all the subjects who participated in the study and all MCC-Spain collaborators (the lists can be found below).
MCC-Spain study group: Maria Teresa Alonso, Pilar Amiano, Cristina Arias, Mikel Azpiri, Yolanda Benavente, Elena Boldo, Aurora Bueno, Mariona Bustamante, Francisco Javier Caballero, Elías Campo, Rafael Cantón, Rocío Capelo, Carme Carmona, Delphine Casabonne, María Dolores Chirlaque, Judith Cirac, Juan Clofent, Enrique Colado, Laura Costas, Marta Crous, Rosa del Campo, Marian Díaz Santos, Trinidad Dierssen-Sotos, María Ederra, Ana Espinosa, Marieta Fernández Cabrera, Ana Fernández Somoano, Tania Fernández Villa, Esther García García-Esquinas, Paloma García Martín, Inés Gómez-Acebo, Cristina González Puga, Esther Gràcia, Marcela Guevara Eslava, Elisabet Guinó, José María Huerta, Virginia Lope, Gonzalo López-Abente, Carlos Lopez-Otín, Begoña Martinez Argüelles, Sergio Merino Salas, Benito Mirón Pozo, Antonio José Molina de la Torre, Eduardo Moreno, Concepción Moreno Iribas, Nicolás Olea, Gemma Osca Gelis, Laia Paré, Miquel Porta, Montse Puig, Manuel Rivas del Fresno, Claudia Robles, Marta María Rodríguez Suarez, Beatriz Romero, Ana Isabel Sáez Castillo, Maria Sala Serra, Dolores Salas Trejo, Ana Santaballa, Miguel Santibáñez, Ángeles Sierra, Ana Souto, Cristina M Villanueva.
BARCELONA
CREAL: Estela Carrasco, Yasmin Sabaté, Cecília Persavento, Mireia García, Glòria Carrasco, Ainara Expósito, H. Mar: Montse Andreu, Xavier Bessa, Mercè Piracés, José Antonio Lorente, Ignasi Tusquets, Inma Collet, Felip Bory, Manuel Pera; Eugènia Abella, Francesc Garcia, Antonio Salar; H. Germans Trias i Pujol (Can Ruti): Marta Piñol, Jaume Fernandez-Llamazares, Marta Viciano Martín, Elisenda Garsot, Luis Ibarz Servio, Montse Arzoz, Luisa Suarez, José Manuel Ruiz. H. Clínic: Antoni Castells, Anna Serradesanferm, Anna Bosch, Montse Muñoz, Montse Fontanillas, Antonio Alcaraz, Lourdes Mengual. PAMEM: Enric Duran; CAP Barceloneta (Barcelona): Clara Izard, Carmen López; CAP Vila Olímpica (Barcelona): Josep Manuel Benítez, Alex Bassa Massanes, Olga Gonzalez Ferrer. Atenció Primària Costa de Ponent: Jesús Almeda, Sònia Sarret; CAP Amadeu Torner (L’Hospitalet de Llobregat): Marifé Alvarez Rodriguez; CAP Jaume Soler (Cornellà): Albert Boada Valmaseda; CAP Mossèn Cinto Verdaguer (L’Hospitalet de Llobregat): Manoli Liceran, Dolors Petitbó; CAP 17 de setembre (El Prat de Llobregat); Badalona Serveis Assitencials: Jordi Ibàñez i Nolla; CAP Nova Lloreda: Sonia Pérez, Susanna Martínez; CAP Eixample: Josep M. Vilaseca, Laura Sebastián
INSTITUT CATALÀ D’ONCOLOGIA (ICO)
ICO_CLL: Paloma Quesada, Guillermo Sequera, Eva Gonzalez Barca, Eva Domingo-Domenech, Ana Oliveira, Esther Alonso, Esmeralda de la Banda, Yasmin Sabater, Marleny Vergara, Ainara Exposito, Teresa Alonso, Isabel Padrol, Joellen Klaustermeier, Yolanda Florencia, Vanesa Camon, Anna Esteban. Colaboradores Clinic _CLL: Elias Campo, Marta Aymerich, Carlos Lopez-Otin, Amparo Muñoz, Yolanda Torralba. Dolores Dot, Santi Mercadal, Josep Sarra.
Unitat Biomarcadors i Susceptibilitat, ICO: Isabel Padrol, Pilar Medina, Carmen Atienza; Cirurgia Digestiva HUB: Sebastiano Biondo, Javier de Oca, Leandre Ferran; Gastroenterología HUB: Francisco Rodriguez-Moranta, Antonio Soriano, Jordi Guardiola; Oncologia Medica ICO: Ander Urruticoechea, Mayca Galan
HUELVA
Rocío Capelo, Marian Diaz Santos, Juan Manuel Banda, Ángela Zumel. Biobanco del Sistema Público de Salud de Andalucía: Anabel Saez. Jose Antonio Garrido, Marina Lacasaña, JL Gómez Ariza, Tamara García, Miguel Ángel García, Miguel Ángel Alba Hidalgo, Manuel Asuero, Juan Bayo, Valle Coronado Vázquez, Francisco Franco, José Luis Gurucelain, María José Robles Frías, Rudolph Van de Haar, Jesús Viñas. H. Juan Ramón Jiménez, Huelva: Mariano Aguayo, Antonio Pereira, Sofía Pérez Gutiérrez, Ricardo Rada, Juan Candón, Juan Domínguez, Manuel Ramos, Guillermo Pedraza, Juan Braulio, Juana Salas, Diego Labrero, David Muñoz, Fátima Barrero, Sonia Delgado, Luís Galisteo, Antonio Camacho. H. Infanta Elena, Huelva: Javier Caballero, Matilde Jiménez Muñoz, Francisco Arredondo, Ramón Linares, Antonio Tejada. H. R. Rio Tinto, Huelva: Manuel Asuero, Javier Delgado Alés, Francisco Franco, María Valle Coronado, María Luisa Sánchez Bernal.
GRANADA
Hospital Universitario San Cecilio. Servicio de Urología: José Luis Mijan Ortiz, Mercedes Nogueras Ocaña. Hospital Universitario Virgen de las Nieves. Servicio de Hematología: Paloma García Martín. Departamento Medicina Preventiva y Salud Pública. Universidad de Granada: Aurora Bueno Cavanillas, Miguel, Eladio Jiménez Mejías, Obdulia Moreno Abril, Rocío Olmedo Requena. Centro de Salud Zaidín Sur, Servicio Andaluz de Salud: José Luis Gastón Morata. Centro de Salud Zaidín Norte, Servicio Andaluz de Salud: Eva Garrido Morales
CANTABRIA
Pilar González Echezarreta, Luis Mariano López López, Mª Mar González Martínez, Paula Picón Sedano, Almudena de la Pedraja Pavón
MURCIA
Dirección General Salud Pública, Murcia: María-Dolores Chirlaque, José-María Huerta y Concepción López-Rojo, Jaime Mendiola. Hospital Morales Meseguer: Enrique Pellicer, José Manuel Egea Caparrós, Emilio García Oltra y Javier Martín Martínez.
VALENCIA
FISABIO-Salud Pública, Valencia: Ana Molina, Vicent Villanueva, Monica, M Jose Miranda, Carolina Abril, Jacobo Martinez, Dolores Salas; Hospital La Fe: Ana Santaballa, Jose Luis Ruiz, Juan Clofent, Marta Ponce, Pilar Noos, Jose Cervera, Adolfo del Val, Angel Segura, Nuria Jiménez, Elena Bellmunt, Ismael Aznar, David Ramos, Teresa Montón, Mª Cruz Solera; Hospital Dr Peset: Eduardo Moreno, Antonio Mora, Nuria Estañ, Natalia Camarasa.; CAP Trinitat: Jose Vicente Solanas; CAP Fuente de San Luis: Jazmin Ripoll, Juana Cantero
ASTURIAS
Instituto Universitario de Oncología de la Universidad de Oviedo: Cristina Arias Díaz, Ana Fernández Somoano, Ana Souto García, Sara María Álvarez Avellón, Mirko Neumann, María José Fernández González, Marta María Rodríguez Suárez, Guillermo Fernández, Begoña Martínez Argüelles, Enrique Colado, Manuel Rivas del Fresno.
GIPUZKOA
Subdirección de Salud Pública de Gipuzkoa: Ander Gómez, Usoa Garín; Ambulatorio de Gros (OSAKIDETZA): Eduardo Tamayo, M. Angeles Rua; C.S. Lasarte (OSAKIDETZA): M. Luz Jauregi H. Universitario Donostia (OSAKIDETZA): Javier Recio, Marta Fernández, Maite Múgica, Juan Pablo Ciria, Elena Guimón, Cristina Adúriz, Adelaida Lacasta, Jose San Francisco, Isabel Alvarez, Jose M. Enriquez-Navascués, Irune Ruiz; Onkologikoa: M. Jesús Michelena, José Antonio Alberro
NAVARRA
Instituto Salud Pública de Navarra: Antonia Martínez Almansa, Leyre Martínez Goñi, María Ibarrola Elizagaray; María Osés Zubiri Apoyo Técnico: Rosana Burgui Pérez; Hospital Virgen del Camino: Servicio de Anatomía Patológica: Dra. Ana María Puras Gil, Dra. María Concepción De Miguel Medina, Dra. Mª Begoña Repáraz Romero, Dra. Ana Yerani Ruiz de Azúa Ciria, Dra. Marta María de Osquia Montes Díaz, Dra. Mª Socorro Razquin Lizárraga, Dra. Yolanda Laplaza Jiménez. Servicio de Aparato Digestivo: Dr. Carlos Enrique Jiménez López, Dra. Susana Oquiñena Legaz, Dr. Raúl Armendáriz Lezaun. Servicio de Cirugía General: Dr. Héctor Ortiz Hurtado, Dr. Mario De Miguel Velasco, Dr. Pedro Armendáriz Rubio, Dr. Fernando Domínguez Cunchillos, Dr. Álvaro Díaz de Liaño Arguelles. Hospital de Navarra: Servicio de Anatomía Patológica: Dr. José María Martínez Peñuela, Dra. Mª Luisa Gómez Dorronsoro. Servicio de Aparato Digestivo: Dr. Fernando Borda Celaya, Dr. David Ruiz-Clavijo García, Dra. Belén González de la Higuera Carnicer. Servicio de Cirugía General: Dr. José Miguel Lera Tricas, Dr. Enrique Miguel Balén Rivera, Dr. Francisco Vicente García, Dr. José Juan Íñigo Noain. Centro de asistencia extrahospitalaria “Príncipe de Viana” Servicio de Enfermería: Esperanza Aranguren Erdozain, Carmen Irigaray Ulibarrena, Julia Goñi Lopeandía. Unidad de Atención al Paciente: María Artieda Caden. Equipo de Atención Primaria “II Ensanche”: Dr. Fernando Aldana Moraza. Dr. Jesús Javier Arana Domench; Dra. Alicia Arza Arteaga; Dra. Karmele Ayerdi Navarro; Dra. Mª Mercedes del Burgo Tajadura; Dr. Fernando Calle Irastorza; Dra. Mª Jesús Esparza Urrisarri; Dra. Berta Flamarique Zubicoa; Dr. Pablo González Lorente; Dr. Pedro Hualte Sevilla; Dra. Mercedes Lázaro Echamendi; Dr. Álvaro Martínez Díaz; Dr. Jesús María Martínez Salaverri; Dr. Francisco Javier Orozco Gorricho; Dra. Mª Luisa Pérez del Valle; Dr. José de Prado Marcilla. Equipo de Atención Primaria “San Juan”: Dra. Mª Luisa Garcés Ducar. Dr. Pablo Aldaz Herce; Dr. José Enrique Ansorena Barasoain; Dra. Isabel Arceiz Campos; Dra. Elena Arina Vergara; Dra. Begoña Churio Beraza; Dr. Luis Fanlo Blasco; Dr. Luis García Díaz; Dr. Jesús García-Falces Larrañeta; Dra. Nuria Goñi Ruiz; Dr. Juan Guijarro García; Dra. Mª Santos Indurain Orduna; Dr. David Iturbe Larena; Dra. María Pardo Fernández; Dr. Francisco Javier Pérez de Ciriza Pejenaute; Dra. Edurne Ridruejo Escuin; Dra. Isabel Ruiz Puertas; Dra. Inés Aranzazu Urtasun Samper; Dra. Mª Eugenia Usúa Sesma; Dra. Mª Josefa Vigata López; Dra. Carmen Zabalza Apestegui.
MADRID
ISCIII: Cristina Linares, Marta Cervantes, Eva Ferreras, Javier García-Pérez, Pablo Fernández-Navarro, Roberto Pastor, Rebeca Ramis, Ángel González; Entrevistadoras: Tamara Ruiz, Viviana Muñoz, Raquel Delgado; Recogida de datos: María Lanza, María Marín; Biobanco: Manuel Posada, Juan Cosmen, Ana Villanueva; Centro Nacional de Sanidad Ambiental: Argelia Castaño, José Antonio Jiménez, Carmen Navarro.
Demométrica: Ana Rin, Gema Díaz, Marta Herreros, Virginia Pedraza, Patricia López, Miguel de la Fuente.
Hospital Universitario La Paz: Gerencia, Sistemas de Información, Admisión y Archivo: Mercedes Fernández de Castro, Javier Sobrino, Rosa Calvo; Consultas externas: Isabel Carrascal, Rosario Bernal; Cirugía general: Alberto Mateo, Damián García-Olmo, Antonio Zarazaga, Miguel Ángel Gombau, Joaquín Díaz, Teresa Gómez, Teresa Sánchez, Paloma de la Quintana; Ginecología: Juan Ordás, Pilar Cuevas, Margarita Sánchez-Pastor; Urología: Javier J. de la Peña, Ángel Tabernero, Natalia Cámara; Anatomía Patológica: David Hardisson, Asunción Suárez, Emilio Burgos, Fco. Javier Alves, María Miguel; Oncología médica: Jaime Feliú, Cristóbal Belda, Pilar Zamora, Argentina Sánchez, Araceli Hernández, Teresa Beato, Ascensión Arroba, Amparo Ballesteros, Soledad Canora, Carmen García, Pilar López, Manuel Romero, Teresa San José, Manuela Espinel; Oncología radioterápica: Ana Mañas, M. Elena Sánchez.
H. Ramón y Cajal: Gerencia, Sistemas de Información, Admisión, Archivo y Atención al Paciente: José Luis Morillo, Miguel Cuchí, M. Jesús García, Juan Manuel Ramos; Cirugía General: Roberto Rojo, Alfonso Sanjuanbenito, Augusto García Villanueva, Miguel Gras, J.L Cabañas, Vicenta Collado, Ignacio Arano, Isaac Capela, Carmen Mojarrieta, Gastroenterología: Beatriz Peñas, Miguel Rodríguez; Oncología Radioterápica: Alfredo Ramos, Sonsoles Sancho, Asunción Hervás, Pilar Moreno; Ginecología: Concha Sánchez, Dolores Rubio, M.J López, Lidia Montoya, Paz Sancho, Lola Rodríguez, Esperanza Durán, Silvia Morel; Urología: Javier Burgos, Ricardo García, Carmen Gómez del Cañizo, Ana Díaz; Anatomía Patológica: Fernando González, Eva Cristóbal, Constantino Barahona, Ricardo García, Silvia Vázquez, Virginia Esteban, Montserrat Pedrera, Javier Martínez; Oncología Médica: Alfredo Carrato, Carmen Guillén Ponce, Andrea Santos-Olmo.
Atención Primaria: Gerencia Área 5: Ricardo Rodríguez Barrientos; Centro de Salud Barrio del Pilar: Gerardo López, Alberto Fernández, Ana B. García, Ana Noriega, M. Rosario Campo, Elisa Varona, Fernando López, M. José Montero, María Teresa Gómez, Pilar Bartibas, Raquel Sanz, Tomasa Montes. Gerencia Área 4: Miguel Ángel Salinero; Centro de Salud Mar Báltico: Margarita Herrero, Mónica Igea, Carmen Calvo, M. Lorena Rodríguez, Carmen Pérez-Pellón, Marta Maestre; Centro de Salud Los Alpes: María Ayuso Agora, María García Martín, Dolores Velázquez, Begoña Fernández, Miriam Castro, Encarnación Ayuso, Raquel Masa, Concha Antelo, Soledad García, Susana Herrero, Ascensión Delgado. Agradecimiento general todos el personal involucrado en estos centros sanitarios, con especial énfasis al personal administrativo de los mismos
GIRONA
Unitat d’Epidemiologia i Registre de Cancer de Girona:Loreto Vilardell, Montse Puig-Vives, Gemma Osca-Gelis, Maria Buxo, Angel Izquierdo, M Carme Carmona-Garcia, Rocio Rodriguez Romanos, Carlota Torner Galindo, Patricia Martí Bargalló, Esther Rodriguez Sanchez, Marc Saez, Rafael Marcos-Gragera. Hospital Universitari de Girona “Dr. Josep Trueta”: Josep Maria Roncero, David Gallardo, Rosa Coll, Ignacio Blanco, Luis Miguel Alonso Ruano y Elena Alvarez Castaño. Hospital Santa Caterina:Joan Melendez Rusiñol, Rocio Jurado Perez, Isaura Marcé Pujol. CAP de Santa Clara: Conxa Bou. CAP de Angles: Gabriel Coll de Tuero, Alba Coll Negre
LEÓN
Juan Pablo de Barrio Lera, José María Cancela Carral, Carlos Ayán Pérez y Marta Elena García Puente, Silvino Pacho Balbuena, Jose María Canga Presa, Jose Antonio Mariño Ramírez, Antonio Álvarez Martínez, Tomás González de Francisco, Tomás González Elosua, Enrique Pastor Teso, Jesús Fernández Fueyo, Oscar Andrés Sanz Guadarrama, María del Amor Turienzo Frade, Maria Luisa De la Hoz Riesco, Julio Juan Sahagún Fernández, Vicente Simó Fernández, Rosario Canseco Fernández, María Victoria Diago Santamaría, Jose Antonio Pedrosa Simón, Ana María González Ganso, Amaya Villafañe Pacho, Santiago Vivas Alegre, Francisco Jorquera Plaza, Begoña Álvarez Cuenllas, Emiliano Honrado Franco, Mercedes Hernando Martín, María Teresa Ribas Ariño, Cristina Díaz Tascón, José Andrés García Palomo, María del Carmen Castañón López, Manuela Pedraza Lorenzo, Isis Atallah González, Florentino González Rivero, Concepción Hernando Román, Eusebio Álvarez Fernández, Tomás Robles Bayón, Amalia García Fernández, Felipe López Municio, Mercedes García de Celis, María José Bravos García, Luis Ángel Fernández Ingelmo, Javier García-Norro Herrero, María José López Carbajo, Teresa Remacha Esteras, Benilde Valcarce Baz, Consuelo Honrubia Baticón, Eduardo Álvarez Baza, Adoración Urdiales Urdiales, Juan Ignacio López Gil, María Antonia Abia López, Elena Carriedo Ule, Carmen Bombín Diez, Ana Isabel Barragán Marín, Miguel Ángel Pérez Bernabeu, Serafín de Abajo Olea, Carlos Vázquez Rojo, María Ángeles Fernández Fernández, Felisa González González. Entrevistadoras: Lidia García Martínez, María del Huerto Trancón Moratiel, Nuria Cuervo Ramos, Sara Prieto Fidalgo, Sonia Santaclara Pérez, Patricia Rubio Coque, Ángeles García González, Ana Belén Delgado Díez.