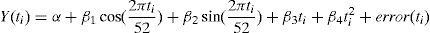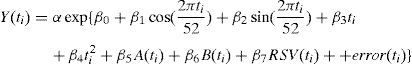To estimate the excess deaths attributed to influenza in Spain, using age-specific generalized linear models (GLM) and the Serfling model for the period 1999-2005.
MethodWe reviewed mortality from influenza and pneumonia and all-cause deaths. We used an additive GLM procedure, including the numbers of weekly deaths as a response variable and the number of influenza virus and respiratory syncytial virus weekly isolates, the population and two variables to adjust for annual fluctuations as covariates. Using the Serfling model, we removed the trend and applied a temporal regression model, excluding data from December to April to account for the expected baseline mortality in the absence of influenza activity.
ResultsGlobally, the excess mortality attributable to influenza was 1.1 deaths per 100,000 for influenza and pneumonia and 11 all-cause deaths per 100,000 using the GLM model. The highest mortality rates were obtained with the Serfling model in adults older than 64 years, with an excess mortality attributable to influenza of 57 and 164 deaths per 100,000 for influenza and pneumonia and all-cause, respectively.
ConclusionsThe GLM model, which takes viral activity into account, yields systematically lower estimates of excess mortality than the Serfling model. The GLM model provides independent estimates associated with the activity of different viruses and even with other factors, which is a significant advantage when trying to understand the impact of viral respiratory infections on mortality in the Spanish population.
Estimar los excesos de mortalidad atribuible a la gripe en España por grupos de edad, usando modelos lineales generalizados (MLG) y modelos Serfling, para el periodo 1999-2005.
MétodoSe revisó la mortalidad por gripe y neumonía y por todas las causas. En el MLG aditivo se incluyó como variable respuesta el número de defunciones semanales, y como covariables el número de aislamientos semanales de virus de la gripe y de virus respiratorio sincitial, la población y dos variables que corrigen las fluctuaciones anuales. En el modelo Serfling se eliminó previamente la tendencia y se aplicó un modelo de regresión cíclica, excluyendo los valores desde diciembre hasta abril, para cuantificar la mortalidad esperada en ausencia de actividad gripal.
ResultadosEl exceso de mortalidad atribuible a la gripe fue de 1,1 defunciones por 100.000 habitantes por gripe y neumonía, y de 11 defunciones por todas las causas usando el MLG. Las tasas de mortalidad más altas se observaron con el modelo Serfling en los mayores de 64 años, con un exceso de mortalidad de 57 y 164 defunciones por 100.000 habitantes por gripe y neumonía y por todas las causas, respectivamente.
ConclusionesEl MLG tiene en cuenta la actividad viral y produce de forma sistemática estimaciones de exceso de mortalidad más bajas que el modelo Serfling. El MLG tiene la ventaja de dar estimaciones independientes asociadas a la actividad de diferentes virus y otros factores, lo cual representa un paso importante cuando intentamos entender el impacto de las infecciones virales respiratorias en la mortalidad de nuestra población.
The number of influenza-attributable deaths is difficult to estimate directly as influenza infection is not usually laboratory-confirmed, and a diagnosis of influenza rarely appears as the main cause of death in death certificates. Additionally, many influenza-related deaths occur in patients with underlying conditions in which signs of influenza infection are already difficult to detect, and the reason for decompensation of the patient's primary illness has been laid-aside.1
Consequently, influenza-related mortality should be quantified indirectly, using models estimating the observed excess deaths in winter seasons above the predicted mortality baseline in the absence of influenza virus circulation.2,3 For many years, the term “influenza-related mortality” or “influenza-associated mortality”4–7 has been widely used in the scientific literature to report figures which reflect the impact of influenza on population mortality. These estimates were normally used to establish the cost-effectiveness and cost-benefit of the various strategies available for the prevention and control of this disease.8,9 These calculations used different sources of data or for cause of death, as well as distinct statistical models. Thus, different authors frequently reported excess pneumonia and influenza deaths,10–12 excess deaths from respiratory and circulatory diseases10,13 or total excess deaths,10–12 depending on the cause of death taken as reference. Similarly, distinct statistical models were used, which did not always include measures of influenza activity.
Among the first models used and never abandoned despite criticism of the methodology,14 was that proposed by Serfling4 in 1963, a classical approach based on a cyclic regression model, which continued to be used by different authors with slight modifications depending on their needs.3,12 When an alternative to these models and reliable information about influenza activity became available, Poisson regression models (or generalized linear models based on Poisson distribution function) started to be used, including not only data on influenza activity but also data on other respiratory viruses (respiratory syncytial virus) as covariables, which could explain influenza-attributable excess deaths.
These methods for indirect estimation of excess mortality have been used in several studies. In the United States, an excess mortality rate of 19.6 per 100,000 inhabitants was found, using all-cause deaths11 as outcome, and 2.4 per 100,000 inhabitants using influenza and pneumonia as causes of death.13,15 In Europe, influenza-associated mortality using all-cause deaths as outcome ranged from an excess mortality rate of 13 per 100,000 inhabitants in Portugal16 to 26 per 100,000 in the Czech Republic.17 With influenza and pneumonia as outcome, reported excess mortality rates associated with influenza were between 1.45 per 100,000 in Portugal16 and 3 per 100,000 in Italy.12 In Spain, all-cause excess mortality attributable to influenza epidemics was estimated in Catalonia as 34.4 weekly deaths among persons aged over 64 years18 (190.79 per 100,000 inhabitants annually).
The aim of this study was to estimate the mortality attributable to influenza in persons over 44 years old during the influenza seasons from 1999-2000 to 2004-2005 in Spain. We also aimed to assess the two different models, with and without measures of influenza activity, to better understand the strengths and weaknesses of the two methods in order to evaluate the burden of influenza disease.
MethodsWe analyzed the national mortality data for the period 1999-2005 provided by the National Statistics Institute, specifying the main cause of death codified according to the 10th International Classification of Diseases (ICD-10). All-cause deaths and the codes of death related to influenza and pneumonia (J09-J18) were taken into account.
Viral activity data (including influenza and respiratory syncytial viruses only) were obtained from the Spanish Influenza Surveillance System (SISS) and the Microbiological Information System (MIS), respectively. The SISS is a sentinel system that aims to provide timely epidemiological and virological information on influenza activity in Spain. The system covers 2% of the total population of the 17 (out of 19) participating Spanish regions. The MIS is a national centralized system in which test results for all infectious diseases, including respiratory syncytial virus are collected, covering 25% of the Spanish population.19 Both viral data corresponded to week 40 of a particular year to week 20 of the following year.
The population data used (annual estimates during the study period) was also provided by the National Statistics Institute.
The presence of the date of death in the examined mortality records allowed deaths to be distributed by epidemiological week during the period analyzed. The viral activity data were also obtained with the same periodicity. The results obtained were displayed by season (from week 40 of a particular year to week 20 of the following year) to take the seasonal pattern of influenza activity into account.
Two types of models were used to estimate the number of influenza-attributable deaths. The first model, the Serfling regression model adapted by Simonsen et al,3,7 did not include viral activity measures. This model, which assumes that errors are distributed according to a normal distribution function, consisted of applying a cyclic regression to the series of weekly mortality rates observed, previously removing the trend. To quantify the expected baseline mortality in the absence of influenza activity, we ran the model, excluding the weekly values between December and April of each year. The weekly excess mortality was calculated as the difference between observed mortality rates and expected mortality rates. The model used was the following:
where Y(ti) is the number of deaths for week ti, two variables to adjust seasonal fluctuations (sine and cosine) and the linear and squared terms in ti.
The second model took measures of viral activity into account. This model was an additive generalized linear model (GLM), which assumes that errors are distributed according to a Poisson distribution function. Its equation included the number of weekly deaths due to the above-mentioned causes as the dependent variable Y(ti), and, as covariables, two variables to adjust seasonal fluctuations (sine and cosine), the linear and squared terms in ti, the number of influenza (A and B types) and respiratory syncytial virus (RSV) isolates per week, and α as a population offset variable:
Excess influenza-attributable deaths were calculated by taking into account the difference between the excess predicted by the model and that obtained when assuming 0 as the number of influenza virus isolates.20 The excess was presented as the absolute number of deaths and mortality rates/100,000 inhabitants.
All of the analyses were done with the statistics program Stata, version 11.
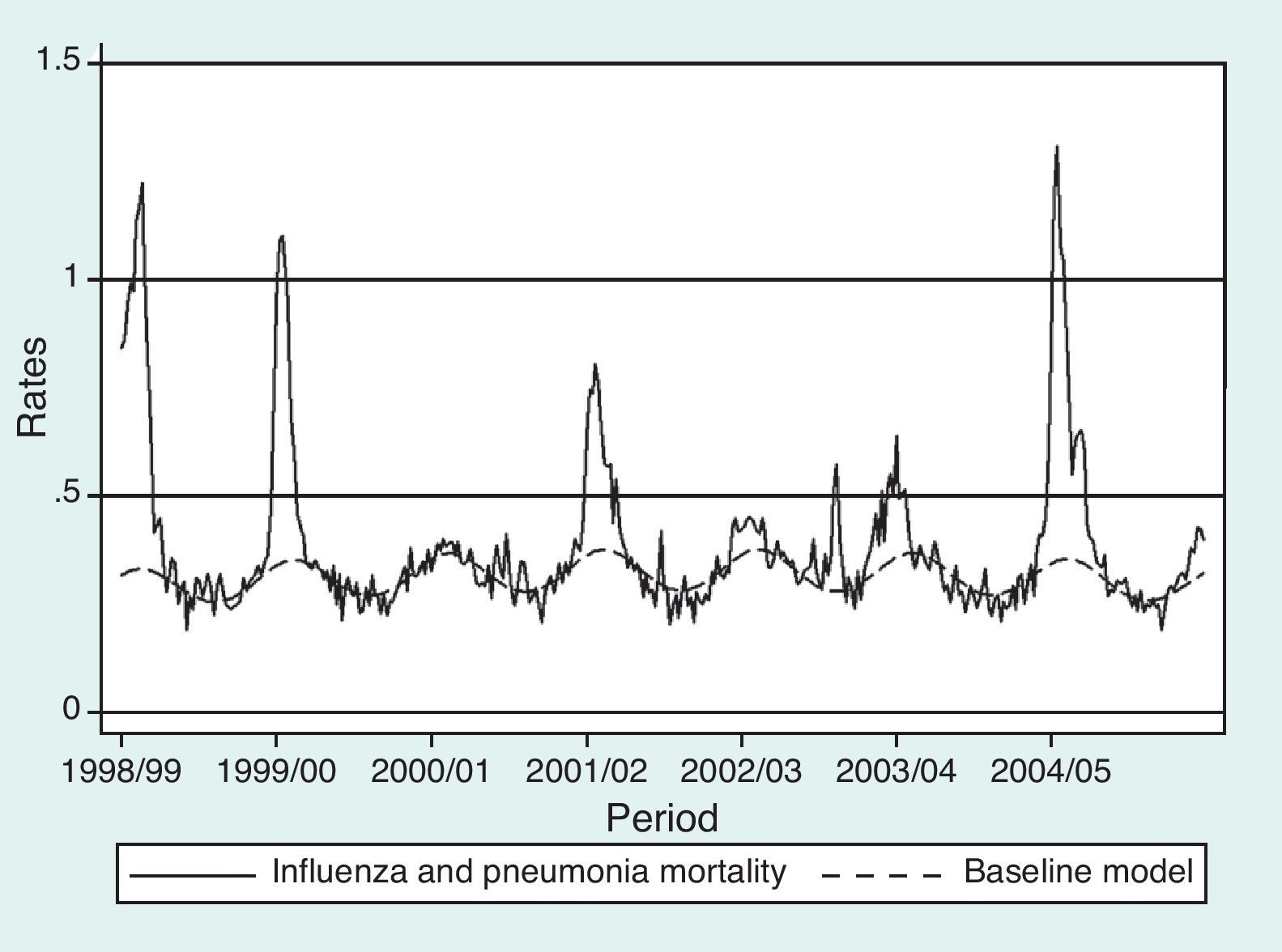
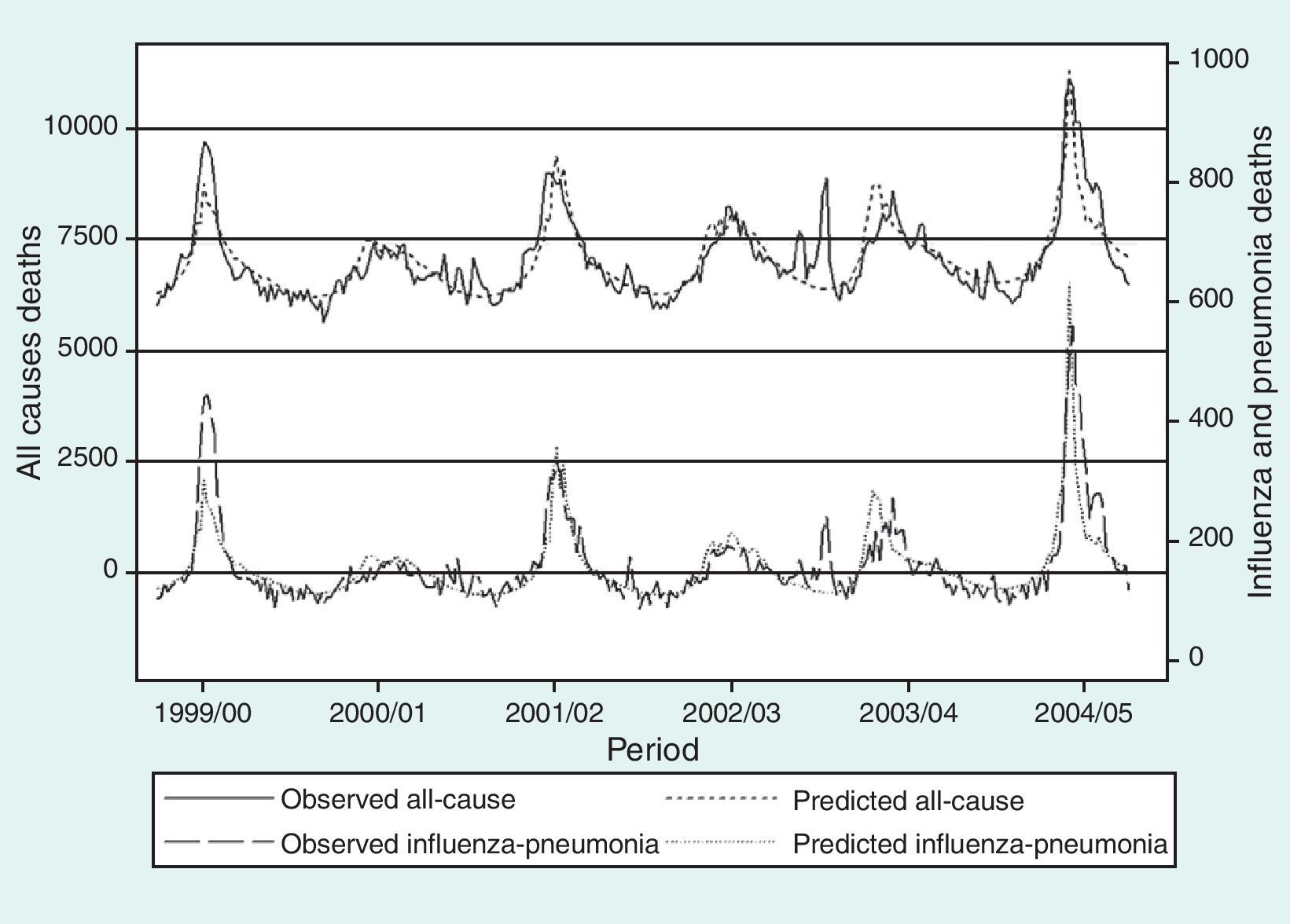
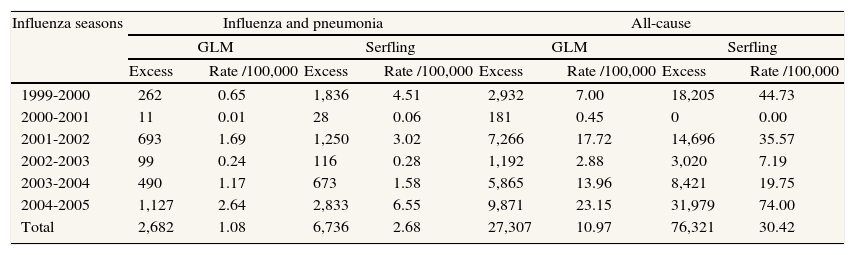
ResultsEstimates of global excess influenza-attributable mortality in Spain, from influenza seasons 1999-2000 to 2004-2005, using the GLM and Serfling models are shown in table 1. The highest mortality rates were observed during the 2004-2005 season (figs. 1 and 2), with an estimated excess mortality rate due to influenza and pneumonia and all-cause deaths of 2.64 and 23.15 deaths per 100,000 inhabitants, respectively using the GLM model, and of 6.55 and 74.00, respectively, using the Serfling model. Conversely, with both models, the lowest excess mortality was found during the 2000-2001 influenza season (table 1).
Influenza and pneumonia and all-cause excess mortality obtained with the generalized linear model and Serfling model in Spain, 1999-2005.
| Influenza seasons | Influenza and pneumonia | All-cause | ||||||
| GLM | Serfling | GLM | Serfling | |||||
| Excess | Rate /100,000 | Excess | Rate /100,000 | Excess | Rate /100,000 | Excess | Rate /100,000 | |
| 1999-2000 | 262 | 0.65 | 1,836 | 4.51 | 2,932 | 7.00 | 18,205 | 44.73 |
| 2000-2001 | 11 | 0.01 | 28 | 0.06 | 181 | 0.45 | 0 | 0.00 |
| 2001-2002 | 693 | 1.69 | 1,250 | 3.02 | 7,266 | 17.72 | 14,696 | 35.57 |
| 2002-2003 | 99 | 0.24 | 116 | 0.28 | 1,192 | 2.88 | 3,020 | 7.19 |
| 2003-2004 | 490 | 1.17 | 673 | 1.58 | 5,865 | 13.96 | 8,421 | 19.75 |
| 2004-2005 | 1,127 | 2.64 | 2,833 | 6.55 | 9,871 | 23.15 | 31,979 | 74.00 |
| Total | 2,682 | 1.08 | 6,736 | 2.68 | 27,307 | 10.97 | 76,321 | 30.42 |
GLM: generalized linear model.
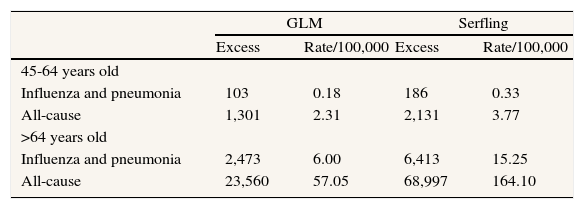
Table 2 shows influenza and pneumonia, and all-cause excess mortality during the study period for the groups aged 45-64 years and 64 or more years, obtained with both models. The highest mortality rates were observed for the group aged 64 years or more, accounting for 6 and 57 deaths per 100,000 inhabitants, attributable to influenza and pneumonia and all-cause, respectively. Values between 2.5 and 2.8 times higher were obtained with the Serfling model.
Influenza and pneumonia and all-cause excess mortality by age group in Spain, 1999-2005.
| GLM | Serfling | |||
| Excess | Rate/100,000 | Excess | Rate/100,000 | |
| 45-64 years old | ||||
| Influenza and pneumonia | 103 | 0.18 | 186 | 0.33 |
| All-cause | 1,301 | 2.31 | 2,131 | 3.77 |
| >64 years old | ||||
| Influenza and pneumonia | 2,473 | 6.00 | 6,413 | 15.25 |
| All-cause | 23,560 | 57.05 | 68,997 | 164.10 |
GLM: generalized linear model.
We estimated an annual overall average of 1,122 influenza-attributable deaths (range: 28 in 2000-2001 to 2,833 in 2004-2005) during the study period using the influenza and pneumonia outcome with the Serfling model, and 447 influenza-attributable deaths (range: 11 in 2000-2001 to 1,127 in 2004-2005) with the GLM model.
DiscussionThe use of two different models to estimate influenza-attributable mortality during a specified period has been fostered by the need to compare the results obtained according to whether viral activity is taken into account or not. Excess influenza-attributable mortality patterns in the different seasons and age groups analyzed were similar with the GLM and Serfling models. However, the GLM model, which takes viral activity into account, yields systematically lower excess mortality estimates than the Serfling model, whose results have already been rated as conservative12,20 as the estimation is based on shorter time periods.
When a model such as the GLM is used, one of the assumptions is that the number of deaths due to a specific virus in any given week is directly proportional to the number of virus detections in that week, independently of the period of the year considered. Additionally, when using an additive model (identity link) the total number of deaths would be the sum of contributions from each virus plus the number of deaths due to other causes.21 For this reason, although the GLM uses a longer time period to estimate the excess than the Serfling model, the higher specificity due to inclusion of viral activity could lead to lower estimates of excess influenza-attributable deaths.
The way in which the viral activity measures are incorporated into the model could also lead to differences. We worked with absolute frequencies of specimens testing positive to the different viruses for which information was available, while some other authors have preferred to consider isolate rates of the different viruses to carry out similar estimates in their own countries.11,22 These authors justified this use by the differences in coverage level and performance of the surveillance systems, factors which can be of particular importance when the period analyzed is long. Working with isolate rates implies knowing how many samples were analyzed, which is not always possible, and does not always guarantee better prediction.23 Nevertheless, working with absolute frequencies of isolates as a measure of the viral activity of different viruses could not have introduced an important bias, because of the good performance of our surveillance system and its constant coverage in the period analyzed.24
Differences in estimates obtained with the two models could also be attributed to the periodicity of the data analyzed (weekly data in our case) and, for the GLM model, could also be due the additive function used in the Poisson distribution. There is some controversy25,26 about the correct use of logarithmical models (log link) to estimate excess deaths, as in these models the number of deaths increases exponentially with the number of isolates, and the effects of each virus on the number of deaths are a multiplicative function, something which is not really permissible. Whether this assumption is accepted or not, what is notable is that additive models such as ours give lower estimates of excess deaths.
We obtained an excess mortality rate due to pneumonia and influenza of 15.3 deaths/100,000 inhabitants in persons aged over 64 years, in reasonable agreement with other countries using the Serfling model12,26 or Poisson model.27 Using the GLM model, we obtained lower estimates of influenza-associated mortality in the same age group than those reported in other studies from the southern hemisphere.28
In both models we used all-cause mortality and “influenza and pneumonia” as more specific causes of influenza-associated deaths. A more specific indicator such as excess deaths due to influenza and pneumonia would be desirable to assess the impact of seasonal influenza epidemics more accurately,18 but would also have some drawbacks. Since not all influenza-associated deaths are coded as “influenza and pneumonia”, the impact of influenza could be underestimated with this indicator.29 In addition, mortality from all causes likely leads to an overestimation of influenza-related deaths. Between the 1999-2000 and 2004-2005 influenza seasons, we estimated an average annual excess of 2,682 and 27,307 influenza-related deaths using influenza and pneumonia or all-cause deaths, respectively. The real impact of seasonal influenza on mortality would be within this range and varies with the season and the predominant influenza virus circulating in each season.
To assess the differences between the magnitude of the two models and to exclude the possibility of residual confounding by seasonality or other factors that could affect our estimates of excess influenza-associated deaths, we carried out an analysis using all deaths from accidents, according to the ICD-10 (V00-Y89), as a control group in the period 1999-2005 (data not shown). The GLM model estimated less than 1% of excess deaths from accidents in all-age groups attributed to influenza, while in the Serfling model this percentage was 4%. These errors might be explained by the clear seasonality of car accidents or by bias introduced by the model in the calculation of excess deaths. These findings suggest that the GLM model might estimate excess deaths attributed to influenza more accurately than the Serfling model.
Despite the differences in mortality estimates obtained with the models used, both models showed the highest influenza-attributable mortality rates for the 2004-2005 season, when the SISS recorded the highest influenza incidence rates since the 1996-1997 seasonal influenza period.30 Likewise, in the 2000-2001 season, which showed the lowest influenza-attributable excess mortality rates with both models, influenza activity did not rise above the baseline threshold for the whole of Europe.30 Influenza-associated mortality can be described by considering the circulating virus strain with both models. As previously reported,13 during seasons with predominant circulation of influenza A(H3N2) viruses (2001-2002, 2003-2004, 2004-2005),30 the average annual excess deaths was higher than in seasons when A(H3N2) was not predominant (1999-2000, 2000-2001, 2002-2003).30
Irrespective of the different results obtained with the two models, which could be important depending on the use made of these results, the GLM model, despite its greater complexity, has the advantage of giving independent estimates associated with the activity of different viruses and even with other measurable factors, which represents an important step when trying to understand the impact of viral respiratory infections on mortality in our population.
The impact of seasonal influenza is usually quantified indirectly, using models estimating the observed excess deaths in winter seasons above the predicted mortality baseline in the absence of influenza virus circulation. Estimates of influenza-associated deaths in Spain are scarce. This study provides estimates of the mortality attributable to influenza in the influenza seasons from 1999-2000 to 2004-2005 in Spain, using two models: one which includes measures of influenza viral activity and another that does not.
What does this study contribute to the literature?The annual overall average for influenza-attributable deaths during the influenza seasons from 1999-2000 to 2004-2005 in Spain, ranged from 447 to 1,122 for influenza and pneumonia causes of death, depending on the method used. This study represents a major improvement in assessing the burden of influenza disease in Spain and provides useful evidence to guide policies for influenza prevention and control.
T. López-Cuadrado, S. de Mateo and A. Larrauri contributed equally to the study design. S. de Mateo wrote the first draft of the manuscript. T. López-Cuadrado carried out the analysis of influenza-related mortality. S. Jiménez and A. Larrauri analyzed the virological information on influenza activity. C. Savulescu contributed to the analysis by measuring the burden of influenza. All authors contributed ideas, interpreted the results, revised the drafts and approved the final version of the manuscript. T. López-Cuadrado is responsible for this article.
FundingThe National Epidemiology Center receives research funding from the EPIA project (funded by MedImmune).
Conflict of interestNone.
We are thankful to all professionals participating in the Spanish Influenza Surveillance System as well as in the Microbiological Information System. We would also like to thank the EPIA project, especially Katy McGuiness and Lone Simonsen, for fruitful discussions and collaborations on modeling strategies to measure the burden of influenza. We are grateful to Caterina Rizzo who shared her experience on the modified Serfling method used in this study. The following persons are Spanish members of the EPIA project: Teresa López Cuadrado, Amparo Larrauri, Salvador de Mateo (National Epidemiology Center, Carlos III Institute of Health, Madrid); Pilar Pérez-Breña, Inmaculada Casas, (National Influenza Center, Carlos III Institute of Health, Majadahonda, Madrid); Raúl Ortiz de Lejarazu (National Influenza Center, Valladolid); Tomás Pumarola (National Influenza Center, Barcelona); and Tomás Vega (Health Sentinel Network of Castilla y León, Valladolid).