The growing number of long-term cancer survivors poses a new challenge to health care systems. In Spain, follow-up is usually carried out in oncology services, but knowledge of cancer survivors’ health care needs in this context is limited. The purpose of this study was to ascertain the health status of long-term survivors of breast, prostate, and colorectal cancer and to characterize their use of health care services.
MethodsRetrospective multicenter cohort study. We collected data from patients’ clinical histories and through telephone interviews, using a specially designed questionnaire that included the SF-36v2 Quality of Life and Nottingham Health Profile scales.
ResultsThe questionnaire was completed by 51.2% (n= 583) of the potential sample. No significant differences were observed between 5-year and 10-year survivors. Overall, more than 80% of respondents were undergoing drug treatment for morbidity related to advanced age. Quality of life was good in most patients, and cancer-related morbidity was low and of little complexity. For the most part, participants reported using primary care services for care of chronic diseases and opportunistic treatment of sequelae related to the cancer treatment. Oncological follow-up was centralized at the hospital.
ConclusionsSurvivors of breast, prostate and colorectal cancer with tumoral detection at an early stage and without recurrences or second neoplasms experienced little morbidity and enjoyed good quality of life. This study proposes exploration of a follow-up model in the Spanish health system in which primary care plays a more important role than is customary in cancer survivors in Spain.
El creciente número de supervivientes de larga evolución de cáncer es un nuevo reto para los sistemas sanitarios. En España, su seguimiento se desarrolla principalmente en los servicios oncológicos y el conocimiento actual sobre sus necesidades sanitarias es en este contexto limitado. El objetivo de este estudio es conocer el estado de salud de los supervivientes de larga evolución en los tumores de mama, próstata y colorrectal y caracterizar el uso de los recursos sanitarios que éstos realizan.
MétodosEstudio multicéntrico de cohortes retrospectivo. Se recogió la información de la historia clínica y de entrevista telefónica a los pacientes mediante un cuestionario específico que incluyó los de calidad de vida SF-36v2 y Perfil de Salud de Nottingham.
ResultadosRespondieron el 51.2% (583) de la muestra potencial. No se observaron diferencias significativas entre los supervivientes entre 5 y 10 años. En conjunto, más del 80% seguían tratamiento farmacológico debido a la morbilidad relacionada con la edad avanzada. La mayoría tenía buena calidad de vida y la morbilidad asociada al cáncer fue reducida y de baja complejidad. Mayoritariamente frecuentan atención primaria para las patologías crónicas y de forma oportunista para las secuelas relacionadas con el tratamiento de cáncer. El seguimiento oncológico está centralizado en el hospital.
ConclusionesLos supervivientes de cáncer de mama, próstata y colorrectal diagnosticados en estadios tempranos, que no han tenido recurrencia ni segundas neoplasias, presentan limitada morbilidad y tienen buena calidad de vida. Este estudio propone explorar en nuestro sistema sanitario un modelo de seguimiento donde la atención primaria tenga un rol más relevante que el actual para la atención de los supervivientes de cáncer en España.
The recent increase seen in cancer survivorship among adults, particularly for those with high-frequency tumors, renders it necessary to analyze these patients’ situations several years after diagnosis in order to assess the adequacy of the health care provided for them. In the last decade, reported 5-year survival rates for all cancers have reached 47.3% in men and 55.8% in women in Europe,1 and in Spain, survival of breast, colorectal and prostate cancer, all of them among the most frequent tumors, has been estimated at 86%, over 50%, and 71.4%, respectively.2 Along with the continuous rise in incidence, the growing number of survivors poses a new challenge to health care systems3 due to the magnitude of the impact on health care services that these represent and the still scant evidence available4,5 on the type of clinical follow-up and their health care needs.6 In this context, a variety of health care models and experiences have emerged, including those in which primary care participation predominates.7,8 Though these have displayed viability from a clinical standpoint, scientific evidence is still too insufficient to segment patient risk vis-à-vis treatment received and the appearance of long-term adverse effects.9
In Spain, the specific needs of long-term survivors have not yet been documented. Accordingly, the main aim of this study was to ascertain and compare the health status between the breast, prostate and colorectal cancer survivors at 5 and 10 years, given the high survival especially in breast tumor, and to characterize their use of health care services in order to generate knowledge that informs decision-making related to health care planning and follow-up for this population.
MethodsWe conducted a retrospective, cohort, multicenter study on adult patients who were diagnosed with breast, colorectal and prostate cancer in 2004 and 1999. The study was undertaken in 2010 at four university tertiary hospitals: the Clinic Hospital, the Parc Sanitari Mar Hospital, the Bellvitge Hospital-Catalonian Institute of Oncology (L’Hospitalet) and the Germans Trias i Pujol Hospital-Catalonian Institute of Oncology (Badalona). The Clinical Research Ethics Committees in each health center approved the study.
The study population was identified through analysis of the Catalonian Hospital Discharge Minimum Basic Data Set (HDMBD) at each hospital and use of the International Classification of Diseases-9th Revision (ICD-9). It included all patients diagnosed with breast, colorectal and prostate cancer for the years 2004 and 1999 who underwent the complete treatment (surgery, chemotherapy, radiotherapy, alone or in combination) at the same health center and gave written, informed consent. Patients were excluded if they had experienced a recurrence, presented another primary neoplasm, or had died.
We drew up a purpose-designed form divided into two sections: C1, to record clinical data obtained from a review of clinical histories, including tumor stage at diagnosis, treatment and adverse effects; and C2, to record data furnished directly by the patients themselves via telephone interview. In this section, the SF-36v2 Health-Related Quality of Life and Nottingham Health Profile (NHP) Questionnaires were used to measure the quality of life. The SF-36v2 incorporates two composite scales, the physical component and the mental component scale, derived from eight domains: physical functioning, role limitations due to physical health problems, bodily pain, general health perception, vitality, social functioning, role limitations due to emotional problems and general mental health. The higher scores indicating better functioning.10 The NHP contains 6 categories: physical mobility, pain, sleep, energy, social isolation and emotional reactions, where the higher score the greater the health problem.11
Specific questions (similar to those used in the Catalonian Health Survey12) were included to evaluate use of and satisfaction with health care resources, while others dealt with life changes, complementary therapies, use of medication and sociodemographic aspects. The form was structured by close-ended questions and validated in a sample of 30 patients drawn from all four hospitals. The average length of interview was 25minutes.
Cases were selected by applying the inclusion criteria to the clinical histories of all diagnoses identified. We recorded the clinical and contact data of the patients selected from section C1 and then phoned them to conduct the interview or alternatively, to set up a subsequent call. An information sheet and informed consent form were mailed to the home of all those who agreed to be interviewed, requiring the prospective interviewees to complete and return both documents in a postage-paid envelope enclosed for the purpose. The interviews were recorded in section C2, and the data were then entered into the database, except for personal identification data, which were disassociated and stored to preserve confidentiality. All data was collected from February to June 2010.
We performed data analyses for the sample by tumor and, in the case of colorectal cancer, by sex, as well as a descriptive analysis of the entire sample. The Chi-square test of independence was used to compare tumor type and sex in the categorical variables. The statistical analyses were performed using the SPSS v15 software program.
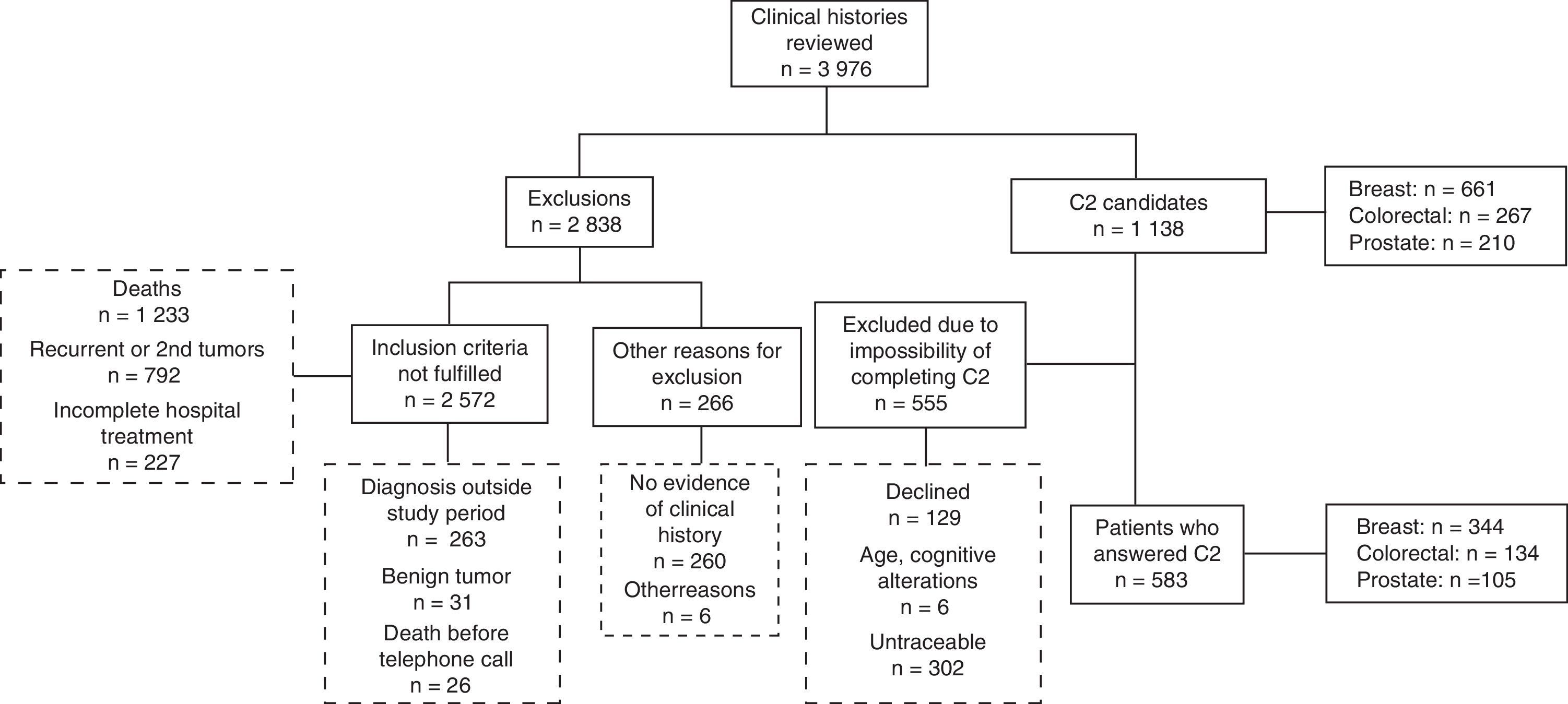
ResultsOf the potential sample, a total of 51.2% (583) responded: 52% of the breast cancer patients; 50.2% of those who had had colorectal cancer; and 50% of those treated for prostate cancer (Fig. 1). No significant differences were observed (p>0.05) between 5-and 10-year survivors; thus, data were aggregated and analyzed by tumor.
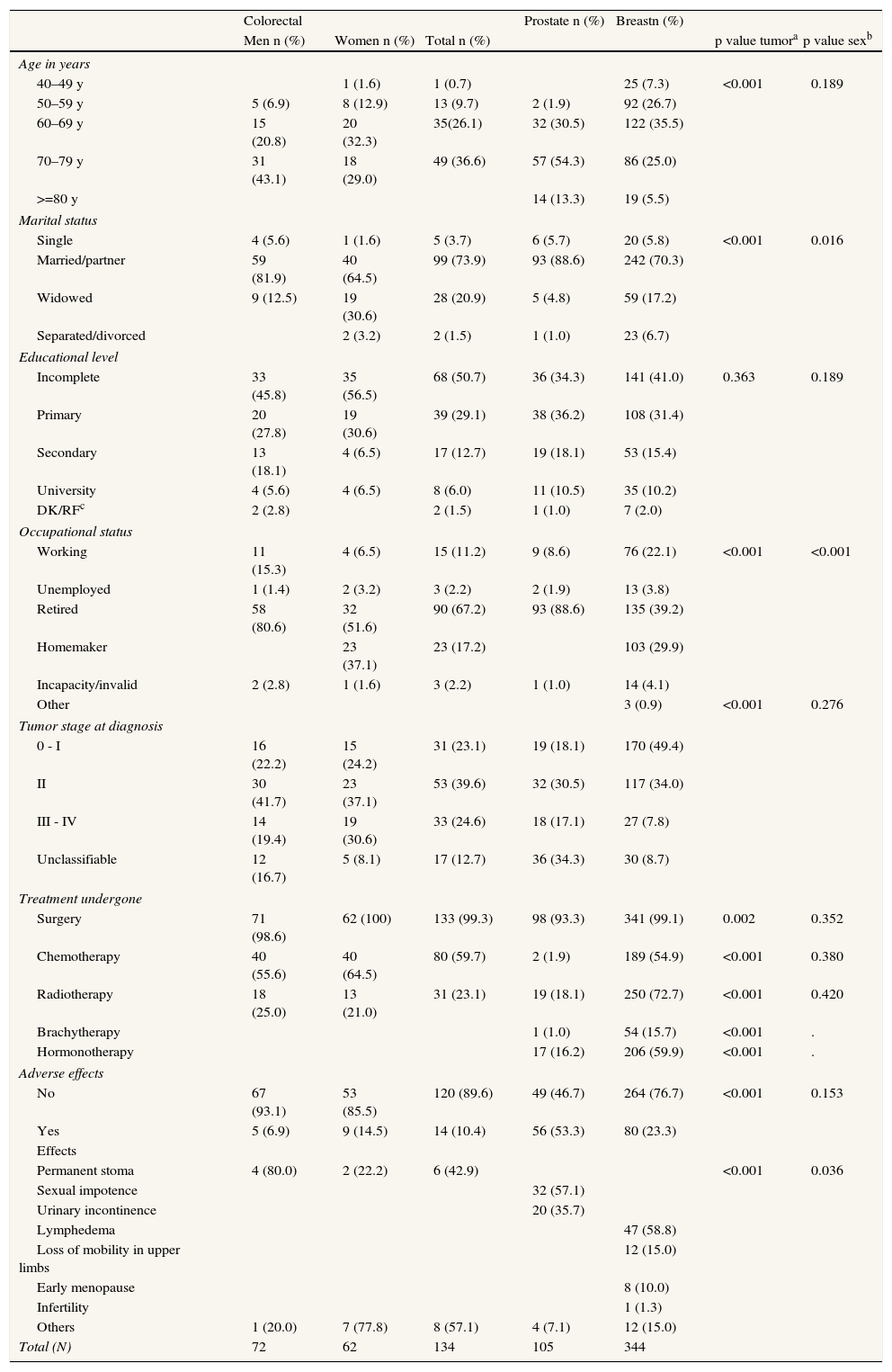
Patients’ sociodemographic and clinical characteristics were also characterized (Table 1). In terms of clinical aspects, it should be noted that most of the patients were diagnosed in the early stages of the disease. Prostate cancer patients presented the most treatment-related sequelae (53.3%) (p<0.001), essentially in the form of sexual impotence and urinary incontinence. The other most frequent long-term effects, although less common, included lymphedema in breast cancer patients and permanent stoma in colorectal cancer patients. Among the patients who did not respond to the questionnaire, tumor stage at diagnosis and treatment types were similar to those observed in participants. Non-participants also experienced the same kinds of adverse effects for each tumor, though less frequently; this population was also characterized by a higher number of patients over 80 for all tumor types respect to the participants (data not shown).
Sociodemographic profile, diagnosis and treatment.
| Colorectal | Prostate n (%) | Breastn (%) | |||||
| Men n (%) | Women n (%) | Total n (%) | p value tumora | p value sexb | |||
| Age in years | |||||||
| 40–49 y | 1 (1.6) | 1 (0.7) | 25 (7.3) | <0.001 | 0.189 | ||
| 50–59 y | 5 (6.9) | 8 (12.9) | 13 (9.7) | 2 (1.9) | 92 (26.7) | ||
| 60–69 y | 15 (20.8) | 20 (32.3) | 35(26.1) | 32 (30.5) | 122 (35.5) | ||
| 70–79 y | 31 (43.1) | 18 (29.0) | 49 (36.6) | 57 (54.3) | 86 (25.0) | ||
| >=80 y | 14 (13.3) | 19 (5.5) | |||||
| Marital status | |||||||
| Single | 4 (5.6) | 1 (1.6) | 5 (3.7) | 6 (5.7) | 20 (5.8) | <0.001 | 0.016 |
| Married/partner | 59 (81.9) | 40 (64.5) | 99 (73.9) | 93 (88.6) | 242 (70.3) | ||
| Widowed | 9 (12.5) | 19 (30.6) | 28 (20.9) | 5 (4.8) | 59 (17.2) | ||
| Separated/divorced | 2 (3.2) | 2 (1.5) | 1 (1.0) | 23 (6.7) | |||
| Educational level | |||||||
| Incomplete | 33 (45.8) | 35 (56.5) | 68 (50.7) | 36 (34.3) | 141 (41.0) | 0.363 | 0.189 |
| Primary | 20 (27.8) | 19 (30.6) | 39 (29.1) | 38 (36.2) | 108 (31.4) | ||
| Secondary | 13 (18.1) | 4 (6.5) | 17 (12.7) | 19 (18.1) | 53 (15.4) | ||
| University | 4 (5.6) | 4 (6.5) | 8 (6.0) | 11 (10.5) | 35 (10.2) | ||
| DK/RFc | 2 (2.8) | 2 (1.5) | 1 (1.0) | 7 (2.0) | |||
| Occupational status | |||||||
| Working | 11 (15.3) | 4 (6.5) | 15 (11.2) | 9 (8.6) | 76 (22.1) | <0.001 | <0.001 |
| Unemployed | 1 (1.4) | 2 (3.2) | 3 (2.2) | 2 (1.9) | 13 (3.8) | ||
| Retired | 58 (80.6) | 32 (51.6) | 90 (67.2) | 93 (88.6) | 135 (39.2) | ||
| Homemaker | 23 (37.1) | 23 (17.2) | 103 (29.9) | ||||
| Incapacity/invalid | 2 (2.8) | 1 (1.6) | 3 (2.2) | 1 (1.0) | 14 (4.1) | ||
| Other | 3 (0.9) | <0.001 | 0.276 | ||||
| Tumor stage at diagnosis | |||||||
| 0 - I | 16 (22.2) | 15 (24.2) | 31 (23.1) | 19 (18.1) | 170 (49.4) | ||
| II | 30 (41.7) | 23 (37.1) | 53 (39.6) | 32 (30.5) | 117 (34.0) | ||
| III - IV | 14 (19.4) | 19 (30.6) | 33 (24.6) | 18 (17.1) | 27 (7.8) | ||
| Unclassifiable | 12 (16.7) | 5 (8.1) | 17 (12.7) | 36 (34.3) | 30 (8.7) | ||
| Treatment undergone | |||||||
| Surgery | 71 (98.6) | 62 (100) | 133 (99.3) | 98 (93.3) | 341 (99.1) | 0.002 | 0.352 |
| Chemotherapy | 40 (55.6) | 40 (64.5) | 80 (59.7) | 2 (1.9) | 189 (54.9) | <0.001 | 0.380 |
| Radiotherapy | 18 (25.0) | 13 (21.0) | 31 (23.1) | 19 (18.1) | 250 (72.7) | <0.001 | 0.420 |
| Brachytherapy | 1 (1.0) | 54 (15.7) | <0.001 | . | |||
| Hormonotherapy | 17 (16.2) | 206 (59.9) | <0.001 | . | |||
| Adverse effects | |||||||
| No | 67 (93.1) | 53 (85.5) | 120 (89.6) | 49 (46.7) | 264 (76.7) | <0.001 | 0.153 |
| Yes | 5 (6.9) | 9 (14.5) | 14 (10.4) | 56 (53.3) | 80 (23.3) | ||
| Effects | |||||||
| Permanent stoma | 4 (80.0) | 2 (22.2) | 6 (42.9) | <0.001 | 0.036 | ||
| Sexual impotence | 32 (57.1) | ||||||
| Urinary incontinence | 20 (35.7) | ||||||
| Lymphedema | 47 (58.8) | ||||||
| Loss of mobility in upper limbs | 12 (15.0) | ||||||
| Early menopause | 8 (10.0) | ||||||
| Infertility | 1 (1.3) | ||||||
| Others | 1 (20.0) | 7 (77.8) | 8 (57.1) | 4 (7.1) | 12 (15.0) | ||
| Total (N) | 72 | 62 | 134 | 105 | 344 | ||
ap value tumor: comparison of distributions by tumor; bp value sex: comparison of distributions by sex; p value: Chi-squared test, cDK/RF: don’t know/refused.
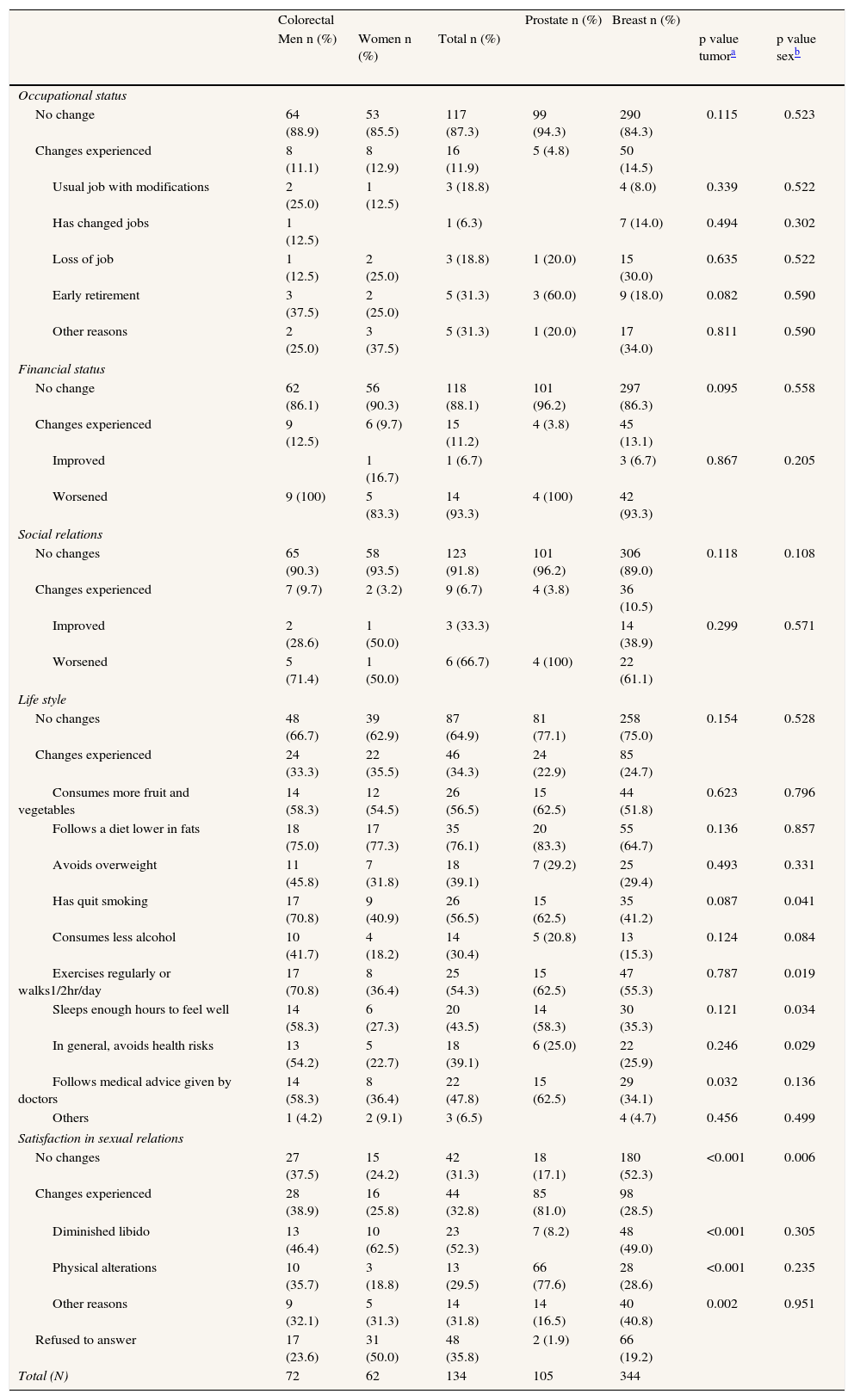
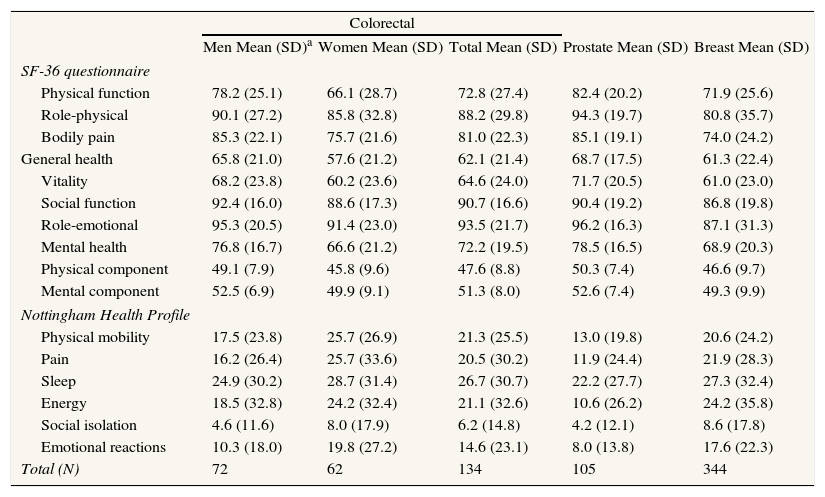
With respect to life changes, most participants had experienced no relevant variations in their occupational or financial status or in their social relations, though it should be stressed that, of the 14.5% of women with breast cancer who reported changes in occupational status, 30% had lost their jobs as a result of the disease (Table 2). In terms of lifestyles, the majority had introduced no changes post-treatment, and among those who had, improved diet and increased physical exercise were the most frequent; no significant variations were observed by tumor type. Insofar as satisfaction with sexual relations was concerned, prostate cancer patients registered the most changes (80%) due to physical alterations and diminished libido, whereas in breast and colorectal tumors the chief cause was a diminished libido (p<0.001) (Table 2). For all patients, the mean score for each of the dimensions of the quality of life (SF-36v2, NHP) by tumor type is presented in Table 3.
Life changes.
| Colorectal | Prostate n (%) | Breast n (%) | |||||
| Men n (%) | Women n (%) | Total n (%) | p value tumora | p value sexb | |||
| Occupational status | |||||||
| No change | 64 (88.9) | 53 (85.5) | 117 (87.3) | 99 (94.3) | 290 (84.3) | 0.115 | 0.523 |
| Changes experienced | 8 (11.1) | 8 (12.9) | 16 (11.9) | 5 (4.8) | 50 (14.5) | ||
| Usual job with modifications | 2 (25.0) | 1 (12.5) | 3 (18.8) | 4 (8.0) | 0.339 | 0.522 | |
| Has changed jobs | 1 (12.5) | 1 (6.3) | 7 (14.0) | 0.494 | 0.302 | ||
| Loss of job | 1 (12.5) | 2 (25.0) | 3 (18.8) | 1 (20.0) | 15 (30.0) | 0.635 | 0.522 |
| Early retirement | 3 (37.5) | 2 (25.0) | 5 (31.3) | 3 (60.0) | 9 (18.0) | 0.082 | 0.590 |
| Other reasons | 2 (25.0) | 3 (37.5) | 5 (31.3) | 1 (20.0) | 17 (34.0) | 0.811 | 0.590 |
| Financial status | |||||||
| No change | 62 (86.1) | 56 (90.3) | 118 (88.1) | 101 (96.2) | 297 (86.3) | 0.095 | 0.558 |
| Changes experienced | 9 (12.5) | 6 (9.7) | 15 (11.2) | 4 (3.8) | 45 (13.1) | ||
| Improved | 1 (16.7) | 1 (6.7) | 3 (6.7) | 0.867 | 0.205 | ||
| Worsened | 9 (100) | 5 (83.3) | 14 (93.3) | 4 (100) | 42 (93.3) | ||
| Social relations | |||||||
| No changes | 65 (90.3) | 58 (93.5) | 123 (91.8) | 101 (96.2) | 306 (89.0) | 0.118 | 0.108 |
| Changes experienced | 7 (9.7) | 2 (3.2) | 9 (6.7) | 4 (3.8) | 36 (10.5) | ||
| Improved | 2 (28.6) | 1 (50.0) | 3 (33.3) | 14 (38.9) | 0.299 | 0.571 | |
| Worsened | 5 (71.4) | 1 (50.0) | 6 (66.7) | 4 (100) | 22 (61.1) | ||
| Life style | |||||||
| No changes | 48 (66.7) | 39 (62.9) | 87 (64.9) | 81 (77.1) | 258 (75.0) | 0.154 | 0.528 |
| Changes experienced | 24 (33.3) | 22 (35.5) | 46 (34.3) | 24 (22.9) | 85 (24.7) | ||
| Consumes more fruit and vegetables | 14 (58.3) | 12 (54.5) | 26 (56.5) | 15 (62.5) | 44 (51.8) | 0.623 | 0.796 |
| Follows a diet lower in fats | 18 (75.0) | 17 (77.3) | 35 (76.1) | 20 (83.3) | 55 (64.7) | 0.136 | 0.857 |
| Avoids overweight | 11 (45.8) | 7 (31.8) | 18 (39.1) | 7 (29.2) | 25 (29.4) | 0.493 | 0.331 |
| Has quit smoking | 17 (70.8) | 9 (40.9) | 26 (56.5) | 15 (62.5) | 35 (41.2) | 0.087 | 0.041 |
| Consumes less alcohol | 10 (41.7) | 4 (18.2) | 14 (30.4) | 5 (20.8) | 13 (15.3) | 0.124 | 0.084 |
| Exercises regularly or walks1/2hr/day | 17 (70.8) | 8 (36.4) | 25 (54.3) | 15 (62.5) | 47 (55.3) | 0.787 | 0.019 |
| Sleeps enough hours to feel well | 14 (58.3) | 6 (27.3) | 20 (43.5) | 14 (58.3) | 30 (35.3) | 0.121 | 0.034 |
| In general, avoids health risks | 13 (54.2) | 5 (22.7) | 18 (39.1) | 6 (25.0) | 22 (25.9) | 0.246 | 0.029 |
| Follows medical advice given by doctors | 14 (58.3) | 8 (36.4) | 22 (47.8) | 15 (62.5) | 29 (34.1) | 0.032 | 0.136 |
| Others | 1 (4.2) | 2 (9.1) | 3 (6.5) | 4 (4.7) | 0.456 | 0.499 | |
| Satisfaction in sexual relations | |||||||
| No changes | 27 (37.5) | 15 (24.2) | 42 (31.3) | 18 (17.1) | 180 (52.3) | <0.001 | 0.006 |
| Changes experienced | 28 (38.9) | 16 (25.8) | 44 (32.8) | 85 (81.0) | 98 (28.5) | ||
| Diminished libido | 13 (46.4) | 10 (62.5) | 23 (52.3) | 7 (8.2) | 48 (49.0) | <0.001 | 0.305 |
| Physical alterations | 10 (35.7) | 3 (18.8) | 13 (29.5) | 66 (77.6) | 28 (28.6) | <0.001 | 0.235 |
| Other reasons | 9 (32.1) | 5 (31.3) | 14 (31.8) | 14 (16.5) | 40 (40.8) | 0.002 | 0.951 |
| Refused to answer | 17 (23.6) | 31 (50.0) | 48 (35.8) | 2 (1.9) | 66 (19.2) | ||
| Total (N) | 72 | 62 | 134 | 105 | 344 | ||
Quality of life.
| Colorectal | |||||
| Men Mean (SD)a | Women Mean (SD) | Total Mean (SD) | Prostate Mean (SD) | Breast Mean (SD) | |
| SF-36 questionnaire | |||||
| Physical function | 78.2 (25.1) | 66.1 (28.7) | 72.8 (27.4) | 82.4 (20.2) | 71.9 (25.6) |
| Role-physical | 90.1 (27.2) | 85.8 (32.8) | 88.2 (29.8) | 94.3 (19.7) | 80.8 (35.7) |
| Bodily pain | 85.3 (22.1) | 75.7 (21.6) | 81.0 (22.3) | 85.1 (19.1) | 74.0 (24.2) |
| General health | 65.8 (21.0) | 57.6 (21.2) | 62.1 (21.4) | 68.7 (17.5) | 61.3 (22.4) |
| Vitality | 68.2 (23.8) | 60.2 (23.6) | 64.6 (24.0) | 71.7 (20.5) | 61.0 (23.0) |
| Social function | 92.4 (16.0) | 88.6 (17.3) | 90.7 (16.6) | 90.4 (19.2) | 86.8 (19.8) |
| Role-emotional | 95.3 (20.5) | 91.4 (23.0) | 93.5 (21.7) | 96.2 (16.3) | 87.1 (31.3) |
| Mental health | 76.8 (16.7) | 66.6 (21.2) | 72.2 (19.5) | 78.5 (16.5) | 68.9 (20.3) |
| Physical component | 49.1 (7.9) | 45.8 (9.6) | 47.6 (8.8) | 50.3 (7.4) | 46.6 (9.7) |
| Mental component | 52.5 (6.9) | 49.9 (9.1) | 51.3 (8.0) | 52.6 (7.4) | 49.3 (9.9) |
| Nottingham Health Profile | |||||
| Physical mobility | 17.5 (23.8) | 25.7 (26.9) | 21.3 (25.5) | 13.0 (19.8) | 20.6 (24.2) |
| Pain | 16.2 (26.4) | 25.7 (33.6) | 20.5 (30.2) | 11.9 (24.4) | 21.9 (28.3) |
| Sleep | 24.9 (30.2) | 28.7 (31.4) | 26.7 (30.7) | 22.2 (27.7) | 27.3 (32.4) |
| Energy | 18.5 (32.8) | 24.2 (32.4) | 21.1 (32.6) | 10.6 (26.2) | 24.2 (35.8) |
| Social isolation | 4.6 (11.6) | 8.0 (17.9) | 6.2 (14.8) | 4.2 (12.1) | 8.6 (17.8) |
| Emotional reactions | 10.3 (18.0) | 19.8 (27.2) | 14.6 (23.1) | 8.0 (13.8) | 17.6 (22.3) |
| Total (N) | 72 | 62 | 134 | 105 | 344 |
a(SD): standard deviation.
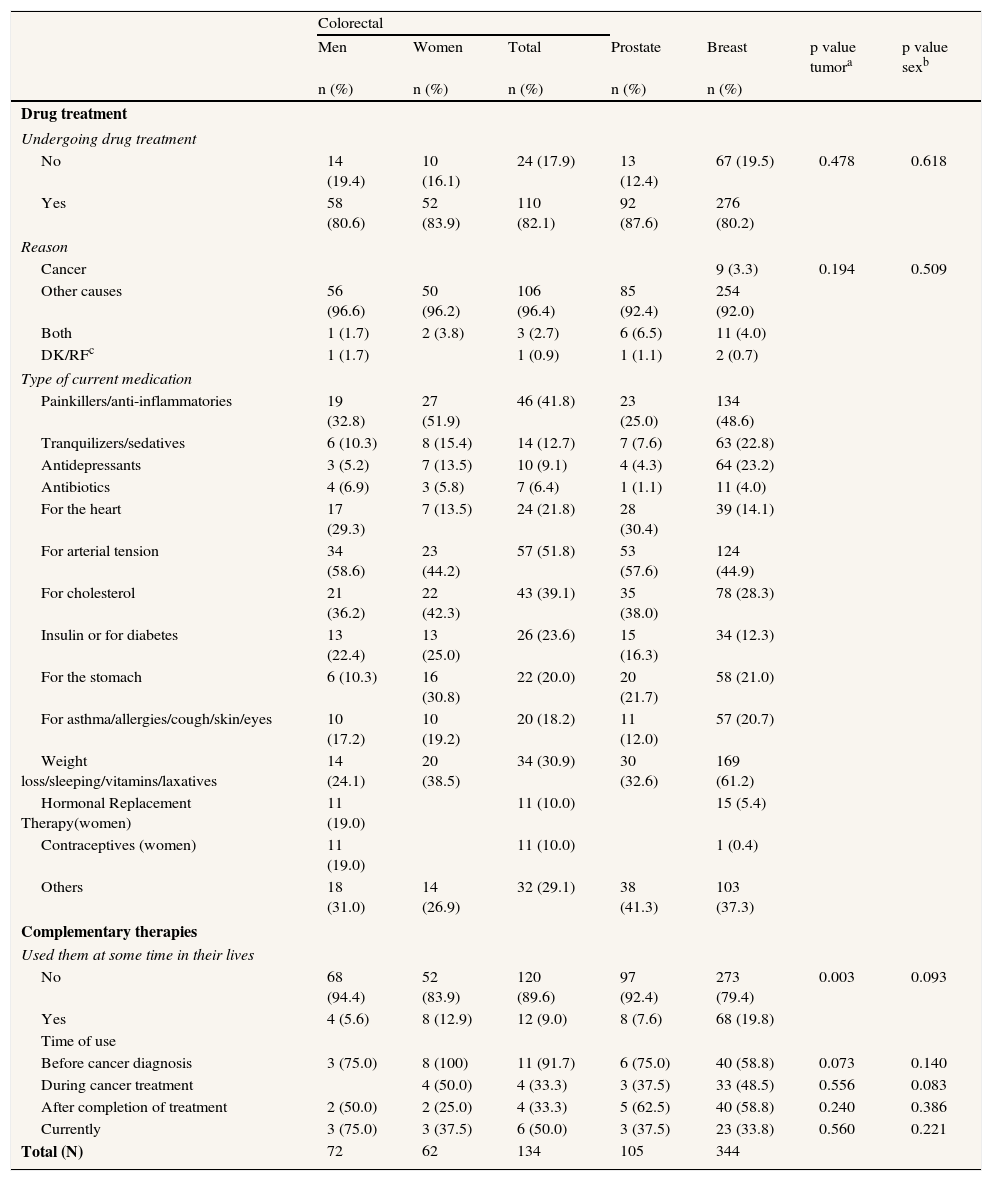
Over 80% of patients for each tumor type were undergoing drug treatment, mainly for arterial hypertension, hypercholesterolemia, pain and cardiological problems (Table 4). Breast cancer patients used tranquilizers and antidepressants more frequently (46%) than their prostate and colorectal cancer counterparts and were also more likely (19.8%) to have used complementary therapies (Table 4). Use of these therapies varied throughout time and disease trajectory, peaking before diagnosis and immediately after treatment but dropping considerably several years after recovery, to levels inferior to those observed prior to diagnosis.
Drug treatment and complementary therapies.
| Colorectal | |||||||
| Men | Women | Total | Prostate | Breast | p value tumora | p value sexb | |
| n (%) | n (%) | n (%) | n (%) | n (%) | |||
| Drug treatment | |||||||
| Undergoing drug treatment | |||||||
| No | 14 (19.4) | 10 (16.1) | 24 (17.9) | 13 (12.4) | 67 (19.5) | 0.478 | 0.618 |
| Yes | 58 (80.6) | 52 (83.9) | 110 (82.1) | 92 (87.6) | 276 (80.2) | ||
| Reason | |||||||
| Cancer | 9 (3.3) | 0.194 | 0.509 | ||||
| Other causes | 56 (96.6) | 50 (96.2) | 106 (96.4) | 85 (92.4) | 254 (92.0) | ||
| Both | 1 (1.7) | 2 (3.8) | 3 (2.7) | 6 (6.5) | 11 (4.0) | ||
| DK/RFc | 1 (1.7) | 1 (0.9) | 1 (1.1) | 2 (0.7) | |||
| Type of current medication | |||||||
| Painkillers/anti-inflammatories | 19 (32.8) | 27 (51.9) | 46 (41.8) | 23 (25.0) | 134 (48.6) | ||
| Tranquilizers/sedatives | 6 (10.3) | 8 (15.4) | 14 (12.7) | 7 (7.6) | 63 (22.8) | ||
| Antidepressants | 3 (5.2) | 7 (13.5) | 10 (9.1) | 4 (4.3) | 64 (23.2) | ||
| Antibiotics | 4 (6.9) | 3 (5.8) | 7 (6.4) | 1 (1.1) | 11 (4.0) | ||
| For the heart | 17 (29.3) | 7 (13.5) | 24 (21.8) | 28 (30.4) | 39 (14.1) | ||
| For arterial tension | 34 (58.6) | 23 (44.2) | 57 (51.8) | 53 (57.6) | 124 (44.9) | ||
| For cholesterol | 21 (36.2) | 22 (42.3) | 43 (39.1) | 35 (38.0) | 78 (28.3) | ||
| Insulin or for diabetes | 13 (22.4) | 13 (25.0) | 26 (23.6) | 15 (16.3) | 34 (12.3) | ||
| For the stomach | 6 (10.3) | 16 (30.8) | 22 (20.0) | 20 (21.7) | 58 (21.0) | ||
| For asthma/allergies/cough/skin/eyes | 10 (17.2) | 10 (19.2) | 20 (18.2) | 11 (12.0) | 57 (20.7) | ||
| Weight loss/sleeping/vitamins/laxatives | 14 (24.1) | 20 (38.5) | 34 (30.9) | 30 (32.6) | 169 (61.2) | ||
| Hormonal Replacement Therapy(women) | 11 (19.0) | 11 (10.0) | 15 (5.4) | ||||
| Contraceptives (women) | 11 (19.0) | 11 (10.0) | 1 (0.4) | ||||
| Others | 18 (31.0) | 14 (26.9) | 32 (29.1) | 38 (41.3) | 103 (37.3) | ||
| Complementary therapies | |||||||
| Used them at some time in their lives | |||||||
| No | 68 (94.4) | 52 (83.9) | 120 (89.6) | 97 (92.4) | 273 (79.4) | 0.003 | 0.093 |
| Yes | 4 (5.6) | 8 (12.9) | 12 (9.0) | 8 (7.6) | 68 (19.8) | ||
| Time of use | |||||||
| Before cancer diagnosis | 3 (75.0) | 8 (100) | 11 (91.7) | 6 (75.0) | 40 (58.8) | 0.073 | 0.140 |
| During cancer treatment | 4 (50.0) | 4 (33.3) | 3 (37.5) | 33 (48.5) | 0.556 | 0.083 | |
| After completion of treatment | 2 (50.0) | 2 (25.0) | 4 (33.3) | 5 (62.5) | 40 (58.8) | 0.240 | 0.386 |
| Currently | 3 (75.0) | 3 (37.5) | 6 (50.0) | 3 (37.5) | 23 (33.8) | 0.560 | 0.221 |
| Total (N) | 72 | 62 | 134 | 105 | 344 | ||
ap value tumor: comparison of distributions by tumor; bp value sex: comparison of distributions by sex; p value: Chi-squared test, cDK/RF: don’t know/refused.
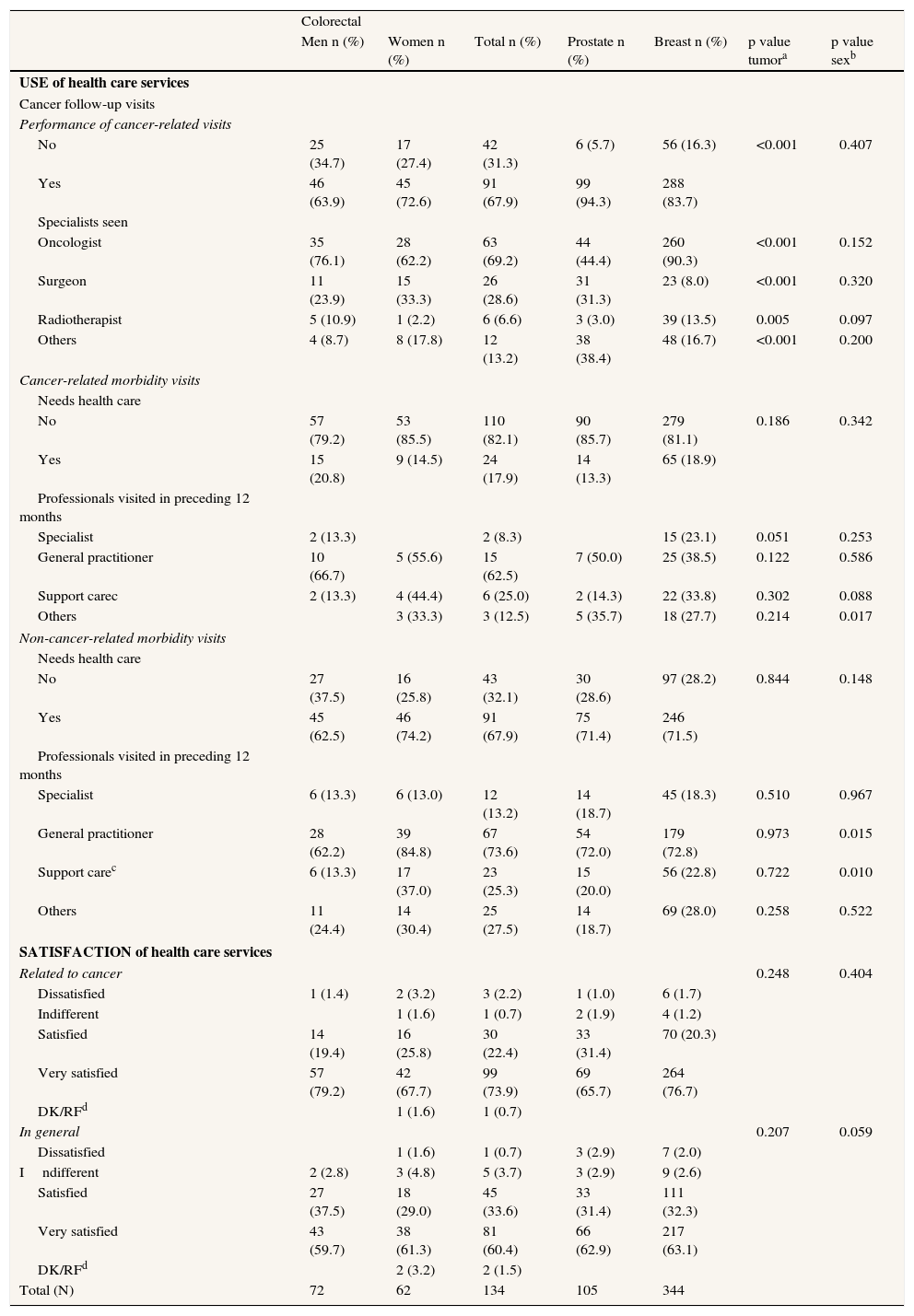
Most patients for all tumor types reported follow-up visits, mainly to the oncologist; visits were more frequent in the case of breast cancer (p<0.001) (Table 5). In the previous 12 months, only a minority used health care services for oncological consultation or treatment, and those who did need medical attention related to cancer generally saw their general practitioner. Other health problems, however, caused close to 70% of sufferers of each type of tumor to see their primary care physician (Table 5). Patients stated that they were satisfied or very satisfied with the health care received, both oncological and in general (Table 5).
Use and Satisfaction of health care services.
| Colorectal | |||||||
| Men n (%) | Women n (%) | Total n (%) | Prostate n (%) | Breast n (%) | p value tumora | p value sexb | |
| USE of health care services | |||||||
| Cancer follow-up visits | |||||||
| Performance of cancer-related visits | |||||||
| No | 25 (34.7) | 17 (27.4) | 42 (31.3) | 6 (5.7) | 56 (16.3) | <0.001 | 0.407 |
| Yes | 46 (63.9) | 45 (72.6) | 91 (67.9) | 99 (94.3) | 288 (83.7) | ||
| Specialists seen | |||||||
| Oncologist | 35 (76.1) | 28 (62.2) | 63 (69.2) | 44 (44.4) | 260 (90.3) | <0.001 | 0.152 |
| Surgeon | 11 (23.9) | 15 (33.3) | 26 (28.6) | 31 (31.3) | 23 (8.0) | <0.001 | 0.320 |
| Radiotherapist | 5 (10.9) | 1 (2.2) | 6 (6.6) | 3 (3.0) | 39 (13.5) | 0.005 | 0.097 |
| Others | 4 (8.7) | 8 (17.8) | 12 (13.2) | 38 (38.4) | 48 (16.7) | <0.001 | 0.200 |
| Cancer-related morbidity visits | |||||||
| Needs health care | |||||||
| No | 57 (79.2) | 53 (85.5) | 110 (82.1) | 90 (85.7) | 279 (81.1) | 0.186 | 0.342 |
| Yes | 15 (20.8) | 9 (14.5) | 24 (17.9) | 14 (13.3) | 65 (18.9) | ||
| Professionals visited in preceding 12 months | |||||||
| Specialist | 2 (13.3) | 2 (8.3) | 15 (23.1) | 0.051 | 0.253 | ||
| General practitioner | 10 (66.7) | 5 (55.6) | 15 (62.5) | 7 (50.0) | 25 (38.5) | 0.122 | 0.586 |
| Support carec | 2 (13.3) | 4 (44.4) | 6 (25.0) | 2 (14.3) | 22 (33.8) | 0.302 | 0.088 |
| Others | 3 (33.3) | 3 (12.5) | 5 (35.7) | 18 (27.7) | 0.214 | 0.017 | |
| Non-cancer-related morbidity visits | |||||||
| Needs health care | |||||||
| No | 27 (37.5) | 16 (25.8) | 43 (32.1) | 30 (28.6) | 97 (28.2) | 0.844 | 0.148 |
| Yes | 45 (62.5) | 46 (74.2) | 91 (67.9) | 75 (71.4) | 246 (71.5) | ||
| Professionals visited in preceding 12 months | |||||||
| Specialist | 6 (13.3) | 6 (13.0) | 12 (13.2) | 14 (18.7) | 45 (18.3) | 0.510 | 0.967 |
| General practitioner | 28 (62.2) | 39 (84.8) | 67 (73.6) | 54 (72.0) | 179 (72.8) | 0.973 | 0.015 |
| Support carec | 6 (13.3) | 17 (37.0) | 23 (25.3) | 15 (20.0) | 56 (22.8) | 0.722 | 0.010 |
| Others | 11 (24.4) | 14 (30.4) | 25 (27.5) | 14 (18.7) | 69 (28.0) | 0.258 | 0.522 |
| SATISFACTION of health care services | |||||||
| Related to cancer | 0.248 | 0.404 | |||||
| Dissatisfied | 1 (1.4) | 2 (3.2) | 3 (2.2) | 1 (1.0) | 6 (1.7) | ||
| Indifferent | 1 (1.6) | 1 (0.7) | 2 (1.9) | 4 (1.2) | |||
| Satisfied | 14 (19.4) | 16 (25.8) | 30 (22.4) | 33 (31.4) | 70 (20.3) | ||
| Very satisfied | 57 (79.2) | 42 (67.7) | 99 (73.9) | 69 (65.7) | 264 (76.7) | ||
| DK/RFd | 1 (1.6) | 1 (0.7) | |||||
| In general | 0.207 | 0.059 | |||||
| Dissatisfied | 1 (1.6) | 1 (0.7) | 3 (2.9) | 7 (2.0) | |||
| Indifferent | 2 (2.8) | 3 (4.8) | 5 (3.7) | 3 (2.9) | 9 (2.6) | ||
| Satisfied | 27 (37.5) | 18 (29.0) | 45 (33.6) | 33 (31.4) | 111 (32.3) | ||
| Very satisfied | 43 (59.7) | 38 (61.3) | 81 (60.4) | 66 (62.9) | 217 (63.1) | ||
| DK/RFd | 2 (3.2) | 2 (1.5) | |||||
| Total (N) | 72 | 62 | 134 | 105 | 344 | ||
ap value tumor: comparison of distributions by tumor; bp value sex: comparison of distributions by sex; p value: Chi-squared test; cSupport care: Psychologist, Physiotherapist, and Nurse; dDK/RF: don’t know/refused.
Our findings suggest that long-term breast, colorectal and prostate cancer survivors who have not experienced recurrence or had second neoplasms enjoy a good quality of life. These results are in line with those reported by other studies13 for this patient profile. Given the early stages observed at diagnosis, these patients had a good prognosis from the outset, also suffering less treatment-related morbidity. Nevertheless, one in four patients presented some permanent treatment-related sequelae, stemming mainly from prostate cancer (sexual impotence and urinary incontinence) and, to a lesser extent, from breast cancer (lymphedema). In the former case, it is important to keep in mind that our study sample was selected on the basis of hospital discharge data, which include patients who were surgically treated but not those who only received brachytherapy or external radiotherapy, a factor which affects the type of morbidity experienced14,15 and accounts for the fact that over half of our patients presented sequelae related to radical prostatectomy.
Most patients in this study were undergoing drug treatment for comorbidities associated with advanced age, including arterial hypertension, hypercholesterolemia, pain and cardiological problems. Considering how infrequently patients accessed psychological and psychiatric services, the use of antidepressants and tranquilizers among women with breast cancer was quite notable, a fact which may indicate underreported psychiatric morbidity addressed exclusively through pharmacological treatment rather than with appropriate specialist care. Moreover, despite the fact that complementary and alternative medicines were seldomly used by patients in general, their use nevertheless tended to be concentrated in breast cancer patients, a common profile reported in the literature by international studies covering patients from Europe16 and elsewhere17 for women with breast cancer. Usage patterns varied across time that was lower some years after treatment than it had been prior to diagnosis; however, this study did not follow individual patient habits, so we do not know if the patients were the same, or if those using complementary therapies after recovery were doing so for the first time. In view of the scant knowledge about the growing use of such therapies, and taking into account that their use could reveal some healthcare needs not properly covered, this is an aspect that warrants greater attention from health care researchers.
Insofar as use of health care services was concerned, despite most patients underwent follow-up at the hospital they did visit the general practitioner for care of cancer-related morbidity. This finding is relevant given that it occurred in a context (Spain) which, despite having a strong primary care system, lacks standardized models for coordinated follow-up between hospital and primary care, although some experiences aimed at improving the relationship between primary health and hospital care.18 This is an area that should clearly be targeted for improvement so as to optimize and coordinate health care follow-up for all the diseases presented by these patients.
In view of the marked degree of satisfaction recorded, a possible explanation for the relatively high use of primary care could be the level of response to their medical needs (including treatment sequelae) which they encountered. This is in contrast to the widespread perception, from the hospital perspective, that patient mistrust would induce rejection of a cancer care follow-up model in which primary care had a more decisive role 19. Our study data, and specifically the satisfaction expressed by the patients themselves, could indicate that patients would not perceive this aspect as a problem. However more research is needed to confirm these findings.
With regard to life changes experienced due to cancer, there were remarkably few in the occupational, financial, and social relations spheres; on the contrary, social functioning was the highest scoring dimension in the quality-of-life questionnaires. In a departure from other studies20,21, it was notable that in ours, the 12.7% of all patients had undergone occupational changes, though it is significant that a small number of patients lost their jobs for reasons related to their disease. The few changes observed could be largely attributable to—and retrospectively associated with—a work-retirement process linked to patients’ advanced age at diagnosis. However, the impact of oncological disease on working life and retirement in Spain requires more qualitative and quantitative research.
Patients reported relevant changes in their sexual relations and, to a lesser extent, in their lifestyles (only 25% had introduced healthy changes in diet and exercise). Given the recent evidence on the benefits of physical activity on risk of recurrence and mortality in breast cancer survivors22 and on the duration and quality of life in long-term survival23,24, this aspect emerges as a specific need requiring intervention targeted to cancer survivors. Sexuality was also identified as an area requiring attention, especially among groups such as prostate cancer patients, among whom physical alterations are a significant component of their disease and treatment. Indeed, over 30% of all study subjects reported difficulties in this dimension, a result that is in line with other studies focusing on these tumor types25,26. It is worth noting that, in general, this is not an area covered by the Spanish healthcare system. These spheres could possibly benefit from an educational and support intervention based on fully integrated nursing care.27
In any case, it is worth underlining the overall high quality of life reported by these patients in comparison to the general Spanish population aged 60 years or older. Indeed, the quality-of-life scores of the survivors in our study exceeded the values in all dimensions28 as well as in the mental component of the SF-36 scale, while physical wellbeing was similar between the two groups.29 The prevalent perception among study subjects of having a better quality of life could stem from the experience of surviving cancer. This aspect is worth exploring in greater depth at a qualitative level in future research.
The results of this study should be assessed in light of certain limitations. Firstly, we used health-related quality-of-life questionnaires in the telephone interview, a methodology that has given rise to conflicting evidence as to whether it is equivalent to self administration30 or if it favors a participant profile with slight physical and mental advantages in comparison to those who complete questionnaires in writing.31 Moreover, some candidates did not participate for reasons of advanced age and/or cognitive alterations. Taken together, these two factors amount to a study limitation. Furthermore, there is no way of knowing if there were clinical or social differences between the patients who could not be located for administrative reasons and those who declined to participate in the study. Finally, the data on adverse effects were obtained by examining clinical histories, a fact which may have led to a slight underestimation of these consequences. Also, the higher proportion of aged non-participants could be explained by the fact that they are dead and we cannot know it.
In conclusion, this study provides a profile of long-term breast, prostate, and colorectal cancer survivors who were diagnosed at an early stage, experienced neither recurrence nor second neoplasms, presented cancer-related morbidity of a low level and of little complexity, and had a good quality of life. For the most part, they reported using primary care services mainly for care of other chronic diseases, as well as for opportunistic treatment of cancer-related sequelae, despite the fact that ongoing oncological follow-up was centralized at a hospital level. The patients stated that they were satisfied with the care received at all health care levels. Emotional needs, sexuality, and healthy lifestyles were identified as specific areas in need of potential care or intervention.
The results of the current study suggest that long-term cancer survivors treated in our health care system could benefit from a comprehensive follow-up approach focusing on health promotion and chronic disease and as well as on surveillance of recurrence and long-term side effects. Therefore, our efforts could be addressed to explore a follow-up model for cancer survivors in which primary care would have an outstanding and integrated role. Further research should be developed so as to confirm our findings and to assess new follow-up care models.
The long-term cancer survivorship is under focus of research given the growing number of survivors and the lack of knowledge on their health care needs. Most of literature is based on Anglo-Saxon experiences. This paper explores the health status of survivors and the use of healthcare resources in relation to the Spanish healthcare system.
What does this study add to the literature?This study gives information on breast, prostate and colorectal cancer survivor's health status and the use of resources within our healthcare system. This allowed for identifying a survivor profile in accordance to quality of life and sequelae issues. This profile could take advantage of a model of follow-up in which primary care would play a relevant role.
Alberto Ruano-Ravina.
Contributions of authorshipThis study was designed by T. Ferro and JM. Borràs. T. Ferro coordinated the field work jointly with M Valverde, MP Fernández and C. Ballano. L. Aliste carried out the statistic analysis. All results were discussed among the authors. T. Ferro wrote the first draft, which was approved by all authors.
FundingThis study has not received financial support.
Conflicts of interestThe authors have no conflicts of interest to disclose.
We would like to thank the Bellvitge University Teaching Hospital, Germans Trias i Pujol University Teaching Hospital, Mar Health Park, Clinical University Teaching Hospital of Barcelona, and the Catalonian Institute of Oncology, as well as their health professionals, without whom this study would not have been possible. Lastly, we would like to express our gratitude for the support received from the Carlos III Institute of Health, a nonprofit organization affiliated with the Ministry of Health in Spain (RD 06/020/0089) within the framework of the Spanish Cancer Research Network (RTICC 06/0089).