“Prescribe Vida Saludable” (PVS) is an organisational innovation designed to optimise the promotion of multiple healthy habits in primary healthcare. It aims to estimate the cost effectiveness and cost-utility of prescribing physical activity in the pilot phase of the PVS programme, compared to the routine clinical practice of promoting physical activity in primary healthcare.
MethodsAn economic evaluation of the quasi-experimental pilot phase of PVS was carried out. In the four control centres, a systematic sample was selected of 194 patients who visited the centre in a single year and who did not comply with physical activity recommendations. In the four intervention centres, 122 patients who received their first physical activity prescription were consecutively enrolled. The costs were evaluated from the perspective of the PVS programme using bottom-up methodology. The effectiveness (proportion of patients who changed their physical activity) as well as the utility were evaluated at baseline and after 3 months. The incremental cost-utility ratio (ICUR) and the incremental cost-effectiveness ratio (ICER) were calculated and a sensitivity analysis was performed with bootstrapping and 1,000 replications.
ResultsInformation was obtained from 35% of control cases and 62% of intervention cases. The ICUR was €1,234.66/Quality Adjusted Life Years (QALY) and the ICER was €4.12. In 98.3% of the simulations, the ICUR was below the €30,000/QALY threshold.
ConclusionsThe prescription of physical activity was demonstrably within acceptable cost-utility limits in the pilot PVS phase, even from a conservative perspective.
Prescribe Vida Saludable (PVS) es una innovación organizativa para optimizar la promoción de múltiples hábitos saludables en atención primaria. El objetivo es estimar el coste-efectividad y el coste-utilidad de la prescripción de actividad física en el pilotaje del programa PVS, respecto a la práctica clínica habitual de promoción de la actividad física en atención primaria.
MétodosSe llevó a cabo una evaluación económica del pilotaje cuasi experimental PVS. En los cuatro centros de control se seleccionó una muestra sistemática de 194 pacientes que visitaron el centro durante 1 año y que no cumplían las recomendaciones de actividad física. En los cuatro centros de intervención se captaron consecutivamente 122 pacientes que recibieron la primera prescripción de actividad física. Los costes se evaluaron desde la perspectiva del programa PVS con la metodología bottom-up. Tanto la efectividad (proporción de pacientes que modificaron su actividad física) como la utilidad fueron evaluadas basalmente y a los 3 meses. Se calcularon la razón de coste-utilidad incremental (RCUI) y la razón de coste-efectividad incremental (RCEI), y se realizó el análisis de sensibilidad con bootstrapping con 1000 repeticiones.
ResultadosSe obtuvo información de un 35% de los casos control y de un 62% de los casos con intervención. La RCUI fue de 1234,66 € por año de vida ajustado por calidad (AVAC) y la RCEI fue de 4,12 €. En un 98,3% de las simulaciones el RCUI estuvo por debajo del umbral de 30.000 €/AVAC.
ConclusionesLa prescripción de actividad física se muestra en unos límites aceptables de coste-utilidad en el pilotaje de PVS, incluso desde una perspectiva conservadora.
Unhealthy habits and the associated risk factors are the most important causes of disease and death in developed countries.1 Evidence shows that 65-80% of cardiovascular disease, 75-90% of type 2 diabetes and 20-30% of all cancers could be prevented if the population were persuaded to adopt a healthy diet, take up physical exercise and stop smoking.2,3 Different studies consistently attribute a 50% decrease in mortality and an eleven year increase in life expectance to the adoption of these habits and moderate alcohol consumption.4–6
We believe that the ideal place to implement this type of interventions that address unhealthy habits is primary healthcare (PHC), for accessibility features and continuity. Despite various interventions have shown their effectiveness,7,8 it is a problem unresolved and healthy lifestyle promotion is far from being integrated in routine primary care practice.9,10
For this reason, in 2008, phase I of the action-research project “Prescribe Vida Saludable” (PVS) was piloted in PHC, in order to set up innovative interventions optimizing the promotion of multiple healthy habits (physical activity, healthy diet, abstinence from smoking) consisting of multiple active ingredients based on main theoretical models of behaviour change and fundamentally, in the 5As strategy (Ask, Advise, Agree, Assist and Arrange follow-up), and modelled by professionals in each intervention centre.11 Professionals in four PHC centres adapted evidence based interventions to their real life context, carrying out organizational changes, including community services (schools, multisport centres, town halls…) and developing new information and communication tools, so that these innovative programmes could be feasible, sustainable and potentially effective. In 2010, phase II was piloted. Its aim was to evaluate the potential feasibility and effectiveness of the PVS in four different reference centres following their usual health promotion practice.
This project is included in the “Strategy to tackle the challenge of chronicity in the Basque Country of the Basque Healthcare Service”: Policy II, prioritize health promotion and disease prevention; Policy III, potentialize the active role of the patient, his responsibility and autonomy; Policy IV, guarantee continued assistance by stimulating multidisciplinary assistance, coordinated and integrated in the different services, levels and sectors of care and Strategic Project 14, clinical professional innovation.
Specifically, the World Health Organization recognizes physical inactivity as one of the principal risk factors for morbidity and mortality.12 The lack of physical activity not only contributes to the increase in prevalence of chronic disease such as cardiovascular disease, obesity, type 2 diabetes, osteoporosis, colon cancer, depression and fall-related injuries, but also contributes to the direct cost of healthcare in developed countries by between 1.5% and 3.0%.13
In the usual clinical practice, general practitioners usually recommend an increase in physical activity, since even a moderate increase has been shown to improve quality of life.14 Nevertheless, the rates and prevalence of physical inactivity remain high.15
The aim of this analysis is to evaluate the efficiency of prescribing physical activity in the PVS programme by means of the estimation of its incremental cost-effectiveness and cost-utility, with respect to the usual clinical practice of promoting physical activity in those who consult for whatever reason.
MethodsAn economical evaluation of the quasi-experimental piloting of PVS from programme perspective was undertaken. In the Basque Country 8 PHC centres participated: 4 control centres, 4 intervention centres. In the intervention centres, 122 patients between 10 and 65 years old who did not meet with the minimum public health physical activity recommendations (at least 30min of moderate physical activity 5 days per week, or at least 20min of vigorous intensity physical activity 3 days per week16) were selected and subsequently received a first physical activity prescription plan between May 2012 and May 2013. In the control centres a systematic sample by age and sex of 194 patients between 10 and 65 years old who did not meet with the minimum physical activity recommendations during the data collection period were selected. Intervention and control patients were measured at baseline and at 3 months. The census-based deprivation index, used as a proxy for the socioeconomic status, was developed for the MEDEA project,17 where the first quintile includes the areas with higher socioeconomic status and the fifth quintile includes areas with low socioeconomic status.
In the intervention centres, having checked the patients’ physical activity, the doctors and nurses provided summary advice and educational materials to the patients and offered an additional appointment in order to prescribe a personalized physical exercise plan. It was assumed that the intervention centres were carrying out the same procedure as the control centres with the addition of the PVS programme promoting physical activity.
CostsThe costs were evaluated from the perspective of the PVS programme, this implied that there were no costs in the control centres since the programme was not deployed. The bottom-up method of collecting cost data was used in the intervention centres. The bottom-up method consists of registering the resources used by each centre and converting them into monetary units.18 To quantify the resources used at patient level, the number of assessment, advisory and prescription of physical activity consultations were counted. The conversion to monetary units was carried out by means of a unit cost for each action: Assessment (A1), Advice (A2), and Prescription (A4).
The unit cost of every action was estimated using the unitary cost of professional intervention (€/minute), the percentage of dedication of each professional to each intervention (%) and the time spent on each intervention (minutes).
The professionals in PHC centres implicated in the PVS programme are administrative assistants, nurses and physicians. In order to calculate the unitary cost of each professional, the gross salary, social security and corresponding structural cost of a Basque Healthcare professional were taken into account.19
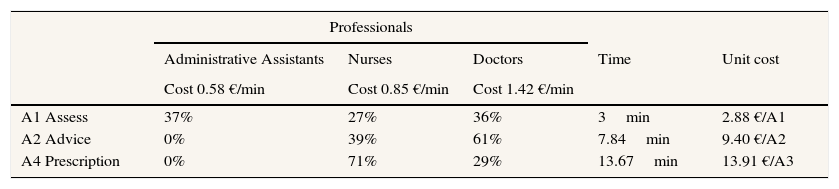
The distribution of professionals in each action appears in Table 1. The dedication to each action of the professionals implicated in the PVS programme was measured using the report of the professionals who participated in A1, A2 and A4. Using a sample of 32 professionals, each action (A1, A2, A4) was timed during three months of the pilot programme in 2010.
Proportion of patients receiving each action of the programme and unitary costs applied in the study.
| Professionals | |||||
|---|---|---|---|---|---|
| Administrative Assistants | Nurses | Doctors | Time | Unit cost | |
| Cost 0.58 €/min | Cost 0.85 €/min | Cost 1.42 €/min | |||
| A1 Assess | 37% | 27% | 36% | 3min | 2.88 €/A1 |
| A2 Advice | 0% | 39% | 61% | 7.84min | 9.40 €/A2 |
| A4 Prescription | 0% | 71% | 29% | 13.67min | 13.91 €/A3 |
The effectiveness was measured by means of a comparison of the people in the pilot study who changed. To measure the change, the 7-day Physical Activity Recall (PAR) questionnaire was used20. The 7-day PAR registered the time spent in all kinds of leisure and occupational activity which lasted more than 10minutes accumulated in the 7 days previous to the appointment, the physical intensity being classified as moderate, vigorous and very vigorous. The proportion of participants who attained the minimum recommended level of physical activity was calculated directly, which are modifiers physical activity habits. The incremental effectiveness was calculated on the difference between the proportion of patients who modified the physical activity habits, between the intervention and control centres.
UtilityHealth-related quality of life scores were obtained using the Spanish version of the Medical Outcomes Trust SF-12 questionnaire21 (version 1), duly filled out by the patients themselves at the start and three months later.21 In the intervention centres, the baseline measurement was made at the time of receiving the first physical activity prescription. The second baseline measurement was applied to those patients who completed the baseline measurement by ordinary post, by recorded telephone message and with the possibility of completing the SF-12 questionnaire by telephone if the patient preferred. In the control centres, the baseline measurement was carried out by post with a telephone message and the possibility of completing the questionnaire by telephone. In the second measurement, the process was repeated with those patients who replied to the baseline measurement. A utility index (SF-6D), based on the method developed by Brazier et al., was calculated as a unique quality of life state for each individual.22 The SF-6D utility index reflects the value, between 0 (death or worst health state) and 1 (perfect health), which society gives to one year of life with a specific health status. It is used to calculate Quality Adjusted Life Years (QALYs), which enabled the numerical representation of health value in a single figure resulting from the combination of people's quantity and quality of life.23 The QALY gained were calculated by adopting the method of the area under the curve.24
AnalysisThe estimate of utility for the comparison of the groups was duly adjusted. The incremental cost-utility ratio (ICUR) quantifies in Euros each existing QALY gained between the intervention group and the control group. The ICUR variable is analyzed using bootstrapping. The bootstrapping method is based on generating multiple replications of the statistics of interest of the sample by substitution of the original data.25 From each of the 1000 simulations produced by bootstrapping, a ICUR composed of the quotient between the incremental cost and utility was obtained. These results were used to obtain a level cost-utility and a curve of acceptability. The cost-utility plane consists in a scatterplot representing the incremental cost and utility for each simulation.26 The acceptability curve is based on the calculation of the percentage of simulations with an ICUR below different threshold values. This percentage is also equal to the probability that the incremental net benefit (INB) is above zero. The curve is obtained when projecting this calculation in function of the threshold value.27
In the centres of intervention and control (1 and 0 respectively) the Incremental Cost Effective Ratio (ICER) is calculated as (C1-C0)/(E1-E0), where C is the average cost per patient and E is the proportion of patients who adopt a healthy habit of physical exercise. In other words, the ICER is the difference of average cost between interventions divided by the difference in effectivity.
The differences in the covariables between the two groups took place by means of the chi-square test and the Fisher's exact test was applied in the case of binary statistics. The Student's t test was used to compare averages between groups. To check if the differences in cost, effectiveness and utility between the two groups were statistically significant, the Student's t test was applied. The t-test is robust to violations of normality and homogeneity, even extreme violations, when the groups size exceeds 50 cases.28 Furthermore, the utility was adjusted by multiple linear regression and the selection of variables used was performed using a backguard technical and maximum likelihood ratio test. At the end the only variables that remained in the model were group and baseline measurement. The statistical analysis of this study was performed using the statistic package SAS 9.2 and Excel.
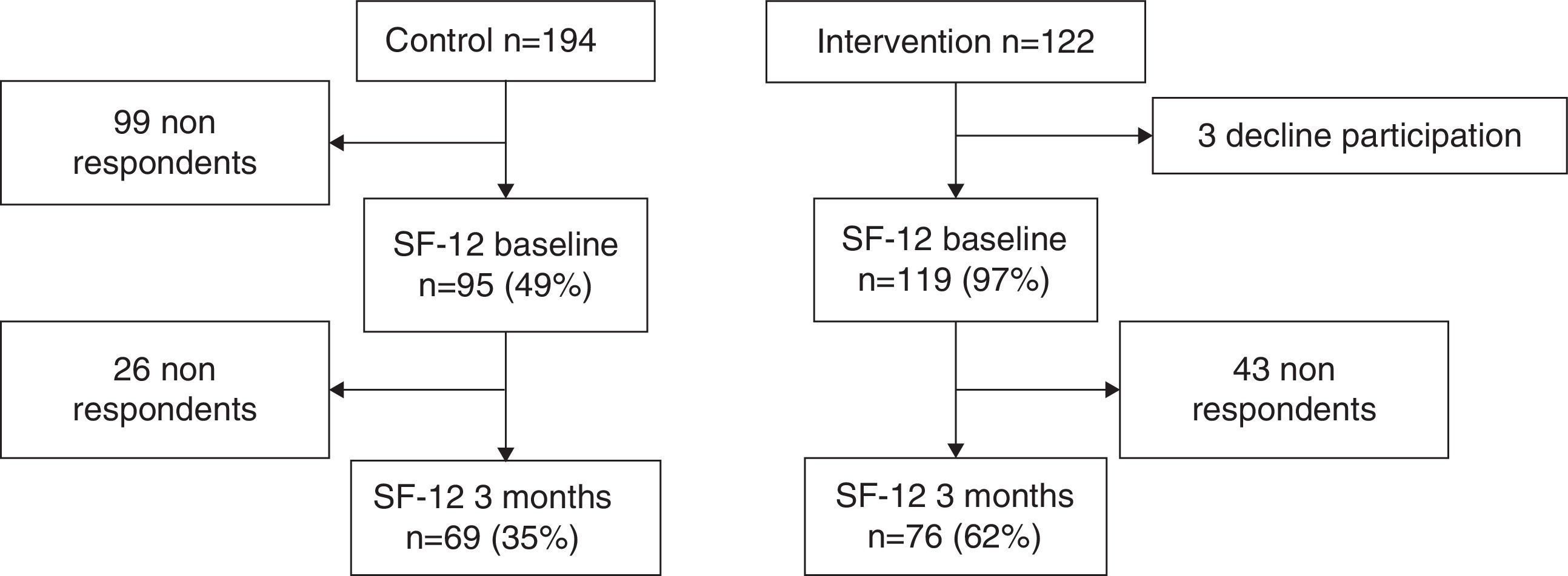
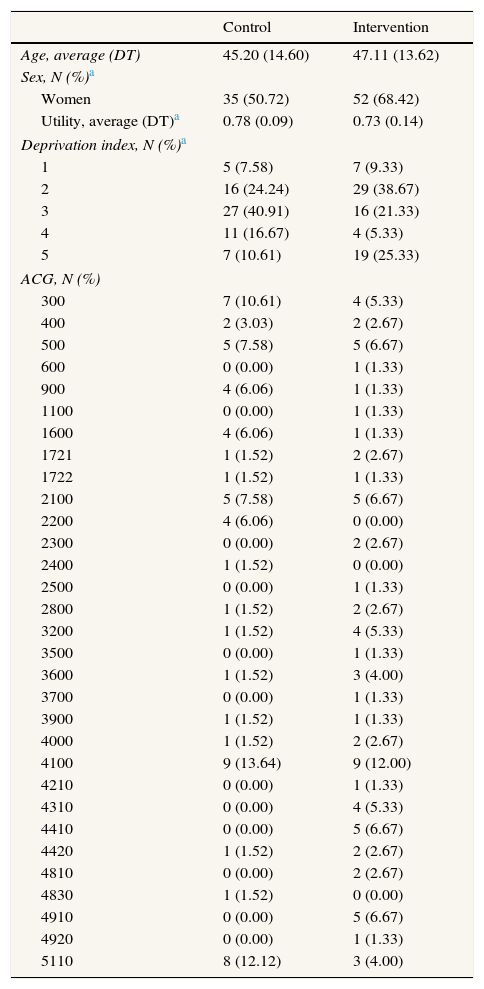
ResultsIn the control centres were selected 194 patients and 122 in the intervention centres. After 3 months, data was obtained from only 69 patients in the control group (35%) and from 76 patients in the intervention group (62%) (Fig. 1). The patients in the control group had an average age of 45 years whereas the average age in the intervention group was 47 years. The proportion of women was higher in intervention than in control, 68.42% and 50.72%, respectively. Statistically significant differences in gender and deprivation exist between the two groups (Table 2).
Baseline characteristics of patients.
| Control | Intervention | |
|---|---|---|
| Age, average (DT) | 45.20 (14.60) | 47.11 (13.62) |
| Sex, N (%)a | ||
| Women | 35 (50.72) | 52 (68.42) |
| Utility, average (DT)a | 0.78 (0.09) | 0.73 (0.14) |
| Deprivation index, N (%)a | ||
| 1 | 5 (7.58) | 7 (9.33) |
| 2 | 16 (24.24) | 29 (38.67) |
| 3 | 27 (40.91) | 16 (21.33) |
| 4 | 11 (16.67) | 4 (5.33) |
| 5 | 7 (10.61) | 19 (25.33) |
| ACG, N (%) | ||
| 300 | 7 (10.61) | 4 (5.33) |
| 400 | 2 (3.03) | 2 (2.67) |
| 500 | 5 (7.58) | 5 (6.67) |
| 600 | 0 (0.00) | 1 (1.33) |
| 900 | 4 (6.06) | 1 (1.33) |
| 1100 | 0 (0.00) | 1 (1.33) |
| 1600 | 4 (6.06) | 1 (1.33) |
| 1721 | 1 (1.52) | 2 (2.67) |
| 1722 | 1 (1.52) | 1 (1.33) |
| 2100 | 5 (7.58) | 5 (6.67) |
| 2200 | 4 (6.06) | 0 (0.00) |
| 2300 | 0 (0.00) | 2 (2.67) |
| 2400 | 1 (1.52) | 0 (0.00) |
| 2500 | 0 (0.00) | 1 (1.33) |
| 2800 | 1 (1.52) | 2 (2.67) |
| 3200 | 1 (1.52) | 4 (5.33) |
| 3500 | 0 (0.00) | 1 (1.33) |
| 3600 | 1 (1.52) | 3 (4.00) |
| 3700 | 0 (0.00) | 1 (1.33) |
| 3900 | 1 (1.52) | 1 (1.33) |
| 4000 | 1 (1.52) | 2 (2.67) |
| 4100 | 9 (13.64) | 9 (12.00) |
| 4210 | 0 (0.00) | 1 (1.33) |
| 4310 | 0 (0.00) | 4 (5.33) |
| 4410 | 0 (0.00) | 5 (6.67) |
| 4420 | 1 (1.52) | 2 (2.67) |
| 4810 | 0 (0.00) | 2 (2.67) |
| 4830 | 1 (1.52) | 0 (0.00) |
| 4910 | 0 (0.00) | 5 (6.67) |
| 4920 | 0 (0.00) | 1 (1.33) |
| 5110 | 8 (12.12) | 3 (4.00) |
ACG: adjusted clinical groups.
The cost data is demonstrated in Table 1. The unitary cost of each action carried out in the PVS programme is 2.88 € for assessment, 9.40 € for advice and 13.91 € for prescription. The average time spent is 3minutes for A1, 7.84minutes for A2 and 13.67minutes for A4. 37% of all professionals engaged in A1 are administrative assistants, 27% are nurses and 36% are doctors. 39% of nurses and 61% of doctors are engaged in A2. And finally, those engaged in A4 are 71% nurses and 29% doctors.
In adjusting the utility of physical activity prescription on utility by age, sex, group, deprivation index and adjusted clinical groups, none of these variables improved significantly the fit of the model to data, only the baseline measurement. Table 3 contains the effectiveness results in the proportion of patients who adopted the healthy habit of physical activity and adjusted utility in QALY of both groups, as well as their costs. The patients in the PVS centres incurred an average cost of 37.04 € (SD: 24.24), while the patients in the control centres didn’t incur any costs because the study was undertaken from the perspective of the PVS programme. The average adjusted utility of the patients PVS is 0.25 QALY (SD: 0.09), while the average adjusted utility of the patients in control centres is 0.22 QALY (SD: 0.04). Using these data, the incremental cost (37.04 €) and the incremental adjusted utility (0.03 QALY) were calculated. The ICUR is 1,234.66 €/QALY and the ICER is 4.12 €. Statistically significant differences in adjusted utility (p-value=0.0310) and in cost (p-value <0.0001) exist between the intervention and control groups.
Incremental cost-utility ratio and incremental cost-effectiveness ratio.
| Cost 0a | Outcome 0a | Cost 1b | Outcome 1b | Incremental cost | Incremental outcome | Ratio | |
|---|---|---|---|---|---|---|---|
| ICUR | 0.00 € | 0.22 QALY | 37.04 € | 0.25 QALY | 37.04 € | 0.03 QALY | 1,234.66 €/QALY |
| ICER | 0.00 € | 24.63% | 37.04 € | 34.21% | 37.04 € | 9.58% | 4.12 € |
ICER: incremental cost-effectiveness ratio; ICUR: incremental cost-utility ratio.
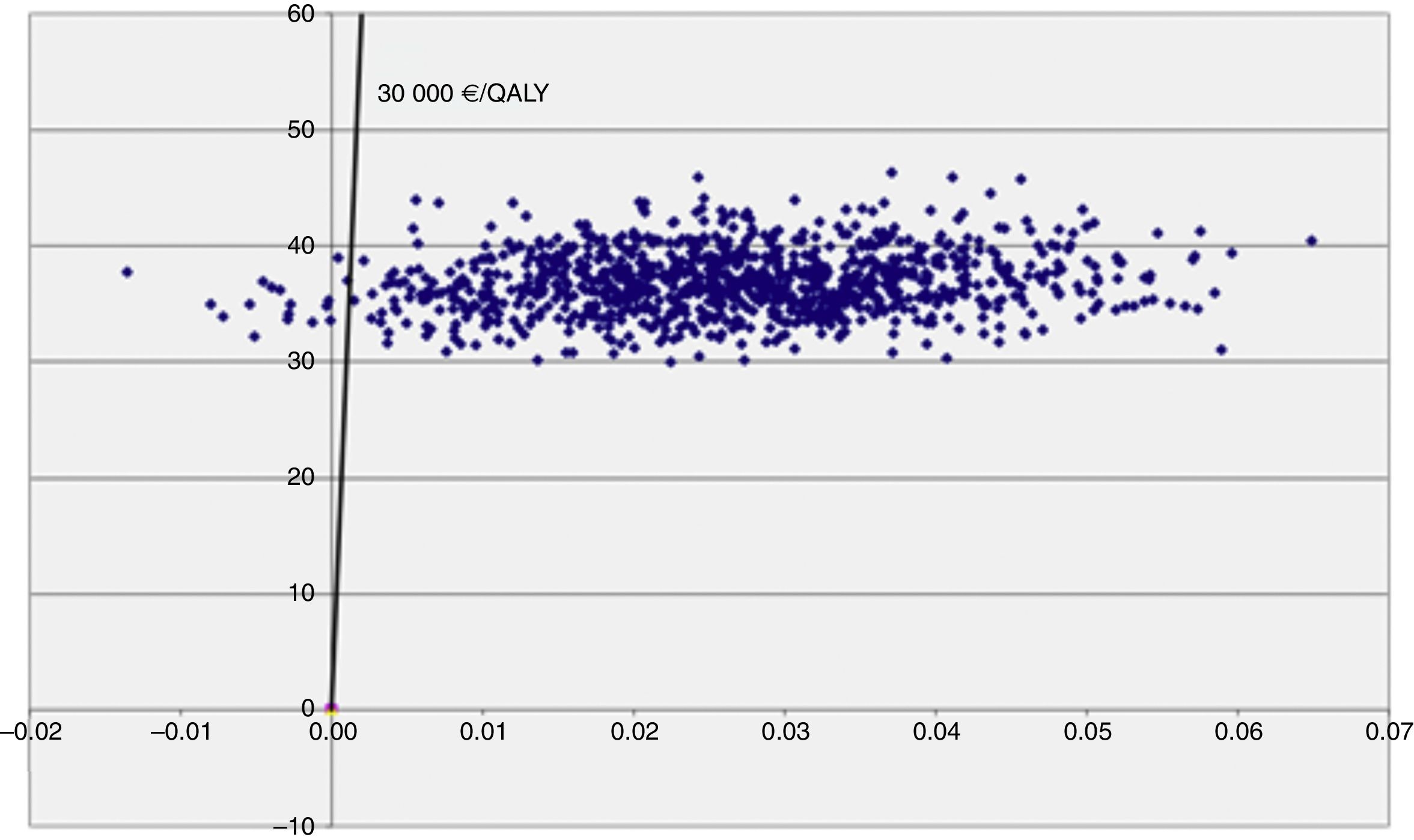
The cost-utility level appears in Figure 2 and enables analysis of the impact of uncertainty of the RCUI. Thus it can be seen that the uncertainty due to a variability of the parameters which affect the utility is much greater than that produced by those which modify the cost.
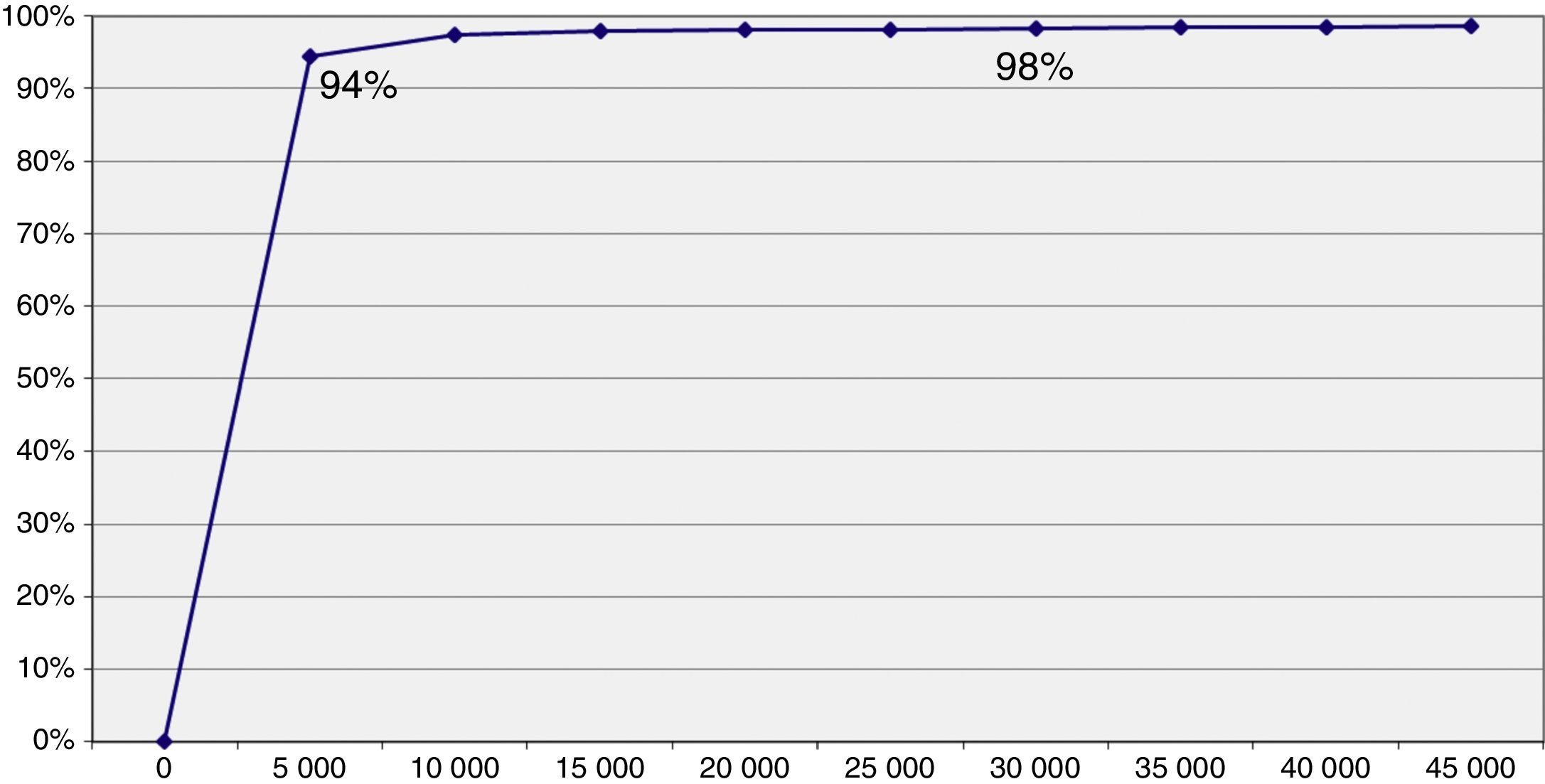
The threshold of 30,000 €/QALY is represented on the cost utility level by means of the straight line which allows us to distinguish the cases where an intervention is cost effective (Fig. 2). The simulations represented by points on the right of said straight line are considered cost effective, while those on the left are not. The acceptability curve is shown in Figure 3 and represents the percentage of simulations which can be seen on the right of the straight line. The percentage of simulations with a result below the threshold of 30,000 €/QALY is 98.3%, while 94% of the simulations satisfy the criteria of acceptability (threshold) of 5,000 €/QALY. Furthermore, we can observe that the acceptability curve never reaches 100% of the simulations. This is due to the fact that the ICUR of some simulations is found in the upper left quadrant where the programme is at its most costly and least effective, since its incremental utility growth is negative.
DiscussionThe prescription of physical activity by means of the PVS innovation thus evaluated presents an efficient cost effective balance in accordance with the results of the pilot scheme in this clinical trial. Both the utility as well as the costs are significantly higher in the intervention group and the ICUR is 1,234.66 €/QALY, far below the 30,000 €/QALY. This estimate is robust in the analysis of additional sensitivity. A large number of the estimates appeared in the quadrant with positive incremental costs and effectiveness.
The economic evaluation manuals show that clinical practice is cost effective when its ICUR is below the threshold.29 In this sense, we have to recognize that in Spain a unanimous criterion on what is an efficient health intervention does not exist, but the threshold of 30,000 €/QALY is considered a frontier between efficient clinical practice and inefficient clinical practice.30 The PVS innovation as method of promoting healthy physical exercise is cost effective with a probability of 98.5% for a threshold of 30,000 €/QALY. The curve of acceptability shows that the uncertainty is low, since more than 94% of the simulations are below the 5,000 €/QALY. These results are better than those of other interventions on healthy lifestyle, with a probability of being cost effective no greater than 93% for thresholds higher than 8,000 €/QALY.31 The differences between groups, principally in gender and deprivation index, mean that the groups are apparently not comparable. We adjusted the analysis by these variables which did not contribute significantly to the model fit, less the baseline measurement.
The professional's dedication to each action is of great importance in the estimation of cost given the perspective of the study. The PVS innovation involves the professionals of the centres in different ways. In spite of the fact that both doctors and nurses carry out A1, it is plausible to consider that A1 could almost entirely be carried out by administrative assistants since they are already the first access point to the centre and at the same time to the PVS programme. Therefore, it is possible that a higher cost is being attributed to this action than necessary.
The rate of replies to the quality of life questionnaire is a limitation of the study. In the intervention centres this rate was 62% compared to 35% in the control centres. It is possible that there is a reply bias in the control centres since those patients who respond to the questionnaire are also the ones with a better quality of life.
In spite of all the previously defined limitations, the results are optimistic when considering the conservative perspective of our analysis and taking into account the results of equivalent studies.32,33 A study with a wider perspective and a longer follow-up period would capture greater improvements in physical activity as well as the prevention of numerous chronic diseases which at the moment involve a heavy care and cost burden. Accordingly, the efficiency would be even greater if we look at it from a larger perspective which includes the QALY attributable to physical activity among the benefits and effectiveness: 3% of discapacity adjusted life years are attributed to physical inactivity per year.34
This study opens the door to a more ambitious study where the effectiveness of the PVS innovation on all the healthy life habits −physical activity, diet, tobacco and alcohol− can be put to the test and this from a healthcare perspective which takes into account the health benefits contained in this kind of preventive intervention. These kinds of interventions have the disadvantage that the huge benefits are not immediately evident and that successful efficacy research not implemented in routine practice of PHC naturally.10 Therefore, the PVS is based on research into implementation.
Studies consistently attribute a 50% decrease in mortality to the adoption healthy life habits. “Prescribe Vida Saludable” is an organizational innovation to optimize the promotion of multiple healthy habits in Primary Healthcare. Nevertheless, is “Prescribe Vida Saludable” efficient by estimating its incremental cost-effectiveness?
What does this study add to the literature?The prescription of physical activity by means of the “Prescribe Vida Saludable” innovation thus evaluated presents an efficient cost effective balance in accordance with the results of the pilot scheme in this clinical trial. This study opens the door to a more ambitious study where the effectiveness of the “Prescribe Vida Saludable” innovation on all the healthy life habits −physical activity, diet, tobacco and alcohol− can be put to the test.
Miguel Ángel Negrín Hernández.
Transparency declarationThe corresponding author on behalf of the other authors guarantee the accuracy, transparency and honesty of the data and information contained in the study, that no relevant information has been omitted and that all discrepancies between authors have been adequately resolved and described.
Authorship contributionsSubstantial contributions to the conception or design of the work: A. Sanz-Guinea and G. Grandes. Acquisition of data: M. Espinosa and C. Martinez. Analysis of data: A. Sanz-Guinea and P. Bully. Interpretation of data: A. Sanchez, H. Pombo, J. Cortada, A. Sanz-Guinea and G. Grandes. Drafting the work: A. Sanz-Guinea and G. Grandes. Revising it critically for important intellectual content: A. Sanchez, C. Martinez, H. Pombo, P. Bully and J. Cortada. All authors reviewed and approved the drafting of the final manuscript.
FundingProject “Evaluación Económica de la Innovación Prescribe Vida Saludable (PVS)”. Expedient: PI13/00573. This study has been funded by Instituto de Salud Carlos III through the project “PI13/00573” (Co-funded by European Regional Development Fund) “Investing in your future”).
Conflicts of interestNone.
We thank the PVS Group.