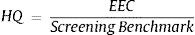The 1st International Conference on Safety and Public Health
More infoThis paper assesses the potential ecological risk of hexavalent chromium (Cr(VI)) and silicon dioxide/silica (SiO2) in well water. Both of pollutants have been classified as carcinogenic compound. The ecological risk assessment of drinking water is an effective tool to evaluate drinking water quality to reduce further risk.
MethodFourteen well water samples were collected around the residential area near cement industrial activity and karst mining area. Estimating the ecological risk of well water which consumed daily evaluated based on Hazard Quotient (HQ) ratio.
ResultsThe mean values of Cr(VI) and SiO2 in well water samples were 0.0017mg/L and 12.94mg/L, respectively. Drinking water in this area are unacceptable. HQ values for SiO2 at all stations are more than 1 (moderate level).
ConclusionsCr(VI) and SiO2 are discoverable within well water surrounding the research location. This finding is used as scientific data and references for ecological protection of drinking water in Maros.
Chromium has two common forms based on oxidation state namely Cr(III) and Cr(VI). Cr(III) are more insoluble (<20μg/L) between pH 7 and pH 10, with a minimum pH solubility of about 1μg/L at pH 8.1 pH values in Cr(VI) tends to be acid in pH 6–7. IARC classifies Cr(VI) as a carcinogenic and harmful substance to humans. The high concentration of chromium in drinking water in Spain can be inferred from piping and fittings tool.2 In Indonesia, a high accumulation of Cr(VI) can be found around the river near the interaction of Talawaan–Tatelu, North Sulawesi.3 Residents exposed to Cr(VI) are confirmed to have digestion, dermatological and hematological abnormalities.4
Silicon dioxide is a dominant minerals and easily discovered in mountains area. This compound may undergo different dissolution or erosion in the environment. Silicon dioxide is a toxic compound and frequently accumulated around the cement plant.5 Inhalation and oral pathways are the primary pathways of human exposure to silicon dioxide. Potential effects of long-term exposure to c-silica include silicosis, COPD and kidney disease. Soluble silicon dioxide in drinking water possibly increase health problems which is correlated to acute gastrointestinal disease, where silicon dioxide was found at high concentrations in hemodialysis patients and kidney disorder.6
The risk level of drinking water is determined based on the HQ (Hazard Quotient) ratio which used to estimate ecological risks of a media.7 HQ values are assessed based on contaminant concentrations in research area and Minimum Risk Level (MRL), a dosage where it should represent long-term effect of contaminants exposure.8 This study aims to assess the ecological risks associated with human consumption of well water containing Cr(VI) and SiO2. The local should aware about long-term effects of drinking water consumption.
MethodStudy areaMaros is an area with a large karst sources. The region is a part of the Bulusaraung Mountains. People is commonly work in agricultural, cement industry, and karst mining. The Maros-Pangkep Karst is a karst region located in South Sulawesi with a surface area of 400km2. The minimum temperature in the research site is 22.8C in July and the maximum temperature is 33.7C in October. The research area is a residential area close to one of the largest cement industries in East Indonesia. Groundwater was used for household and drinking purposes. It is therefore essential to determine its chemical composition periodically.
Spatial distribution in this research were performed with ArcGIS 10.8 and Google Earth Pro by the interpolation method. The tools used to obtain the exact coordinate points by using GPS Garmin 64. The main areas in this study were Kecamatan Lau (Mattiro Deceng), Kecamatan Bontoa (Salenrang), and Kecamatan Bantimurung (Baruga, Tukamasea, Bungaeja, Leang-Leang, Mattoangin, and Manarang).
ProceduresSampling was carried out for 2 days in 5–6 May, 2020. A total of 14 well water samples were taken using HDPE (High-Density Polyethylene) bottle and collected directly from resident wells. The samples were using pre-labeled and pH of is measured in situ using the pH meter ATC-PH2011. The samples were analyzed using the SNI (Indonesian National Standard) 06-2477-1991 guidelines for silicon dioxide and 6989.71:2009 for hexavalent chromium. For the determination of silicon dioxide levels, each sample had a volume 50mL of well water samples added by 50mL and 1mL of HCl 1:1 and 2mL of ammonium molybdate. Ease the mixture for 5min and added by oxalic acid. The sample measured using the PinAAcle 900H Atomic Absorbance Spectroscopy (AAS) PerkinElmer instrument at wavelength 410nm.
Heavy metal analysis need to acidified and digested concentrated acid immediately before analysis until reach a pH<2 with 69% HNO3. This method is used to avoid the presence of precipitating base salts on the sample bottles that possibly reducing the metal concentration. The samples were first filtered. Filtrates were piped by 2.5mL and dissolved by 2 drops of H3PO4, 2mL H2SO4 2M, 0.5mL of diphenylcarbazide, and 25mL aquabidest in volumetric flask. The sample measured its absorbance with a UV–vis. Laboratory quality control was using a Standard Reference Material (SRM 1646a estuarine sediment) from the Department of Commerce, NIST, Gaithersburg, MD 20899 with 3 replications.
Assessment methodRisk assessment approach is used to describe health-related value for toxic compound in drinking water, food, or air sample. The highest possible dose of Cr total in consumable drinking water is 0.003mg/kg/day.8,9 The potential risk resulting from the accumulation of contaminants in environmental media may be determined by the Ecological Risk Assessment (ERA). Estimated risk expressed in the Hazard Quotient ratio is as follows:
EEC (Estimated Environmental Concentration) is the actual concentration of contaminants in soil and water. NOAEL or Screening Benchmark is highest exposure level with no biologically increases of adverse effect. The reference threshold (minimum) based on animal testing for health effects on receptors, in this case, humans. If the value is HQ<0.1; no hazard exists, HQ 0.1–1; low risk, HQ 1–10; moderate risk, HQ>10, high risk.7 The critical health effect of chromium in drinking water is the hyperplasia of small intestinal diffuses that are the most sensitive endpoints and precursors to tumor formation.10
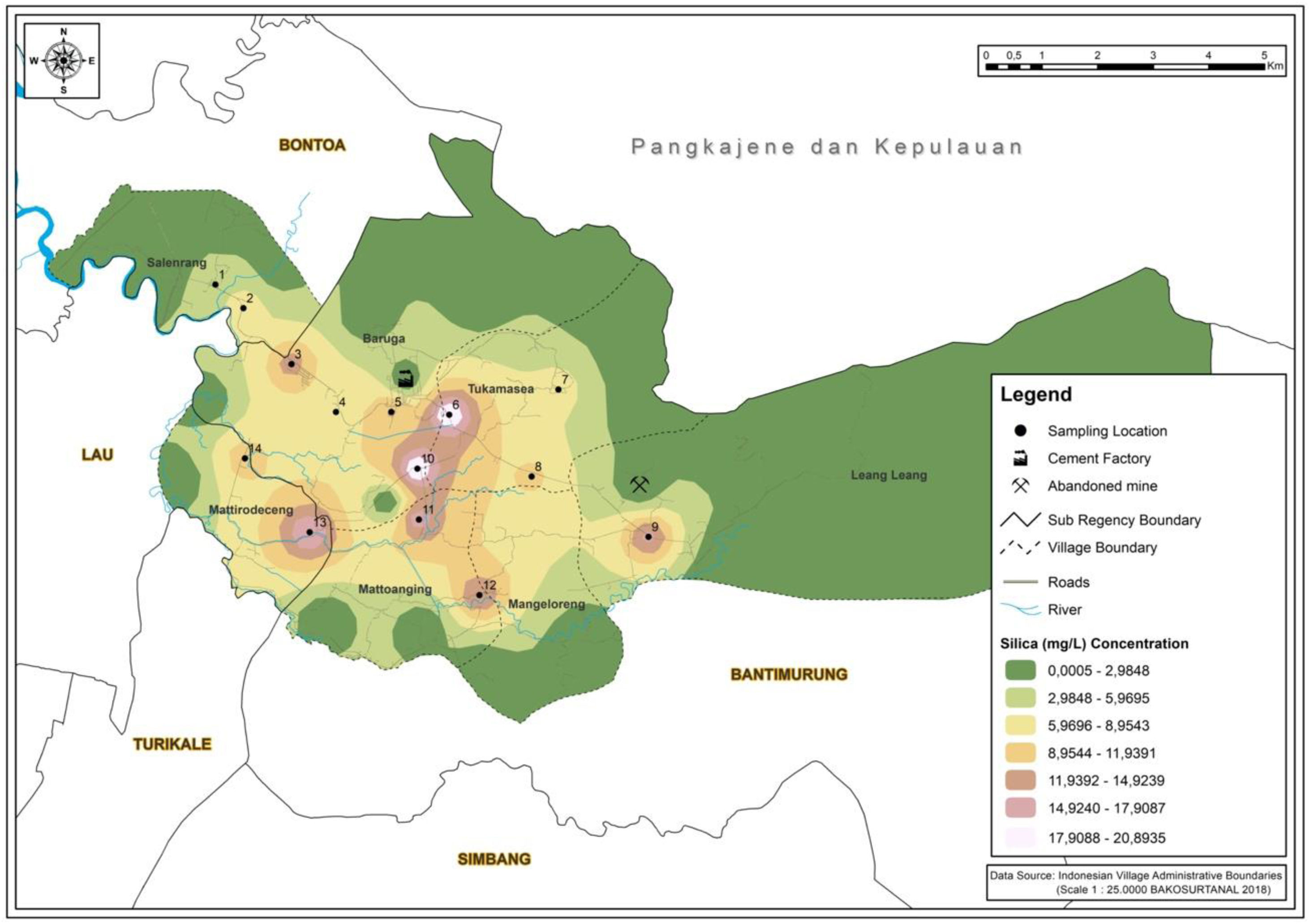
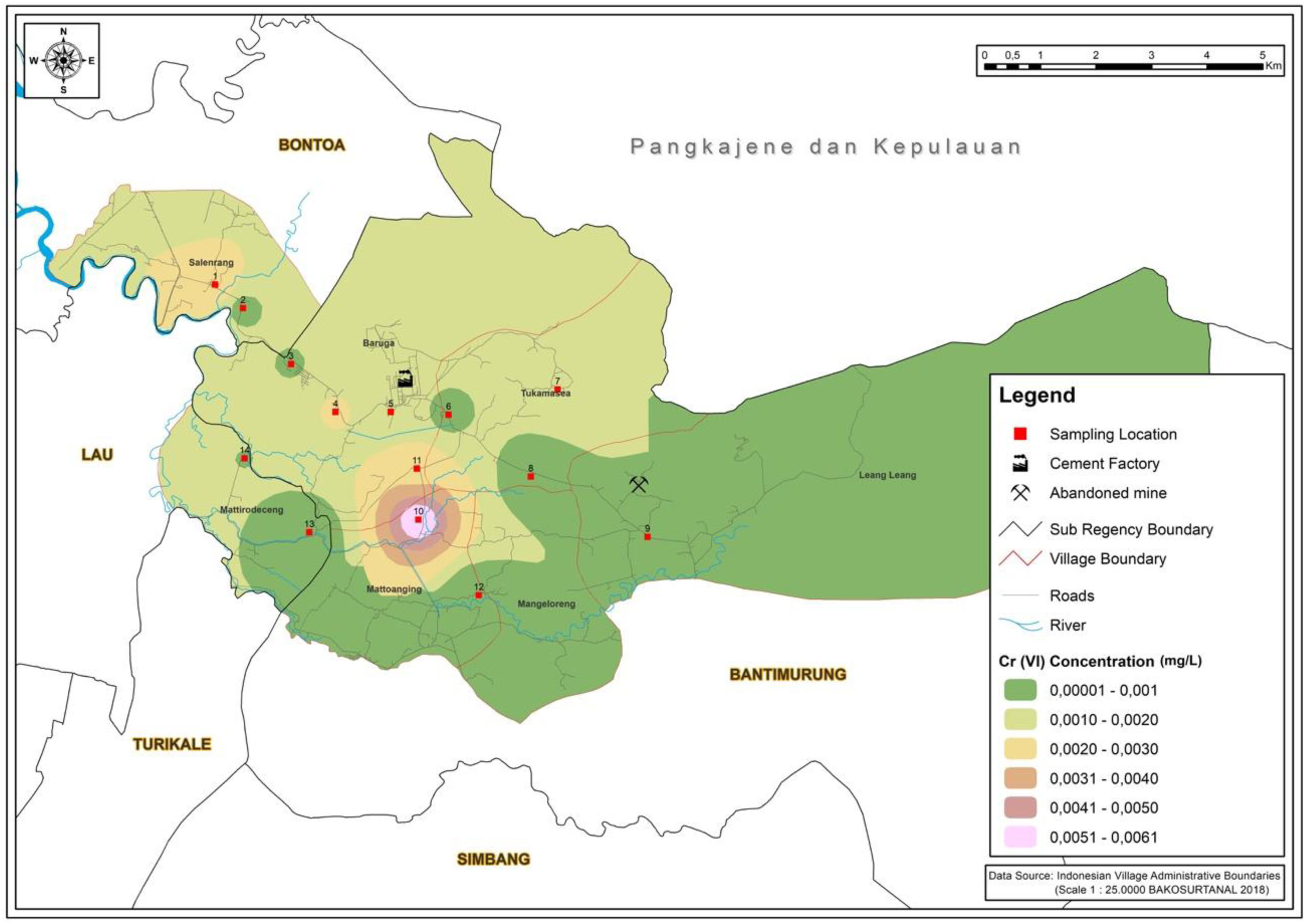
ResultsMapping areaIn Fig. 1, the highest concentration of silicon dioxide is located in station 6. This result data owing to silicon dioxide constitutes as one of the majority compounds in the Karst mountains.11 The concentration of silicon dioxide in groundwater varies between 1 and 30mg/L.12 The value of silicon dioxide is critical in understanding the origin of groundwater and geogenic or anthropogenic processes that may affect the distribution of ions. As showcased in Fig. 2, the highest Cr (VI) concentration rate occurred in station 10. This area is an agricultural region in Manarang village in Tukamasea. It is 3 kilometers away from the cement factory.
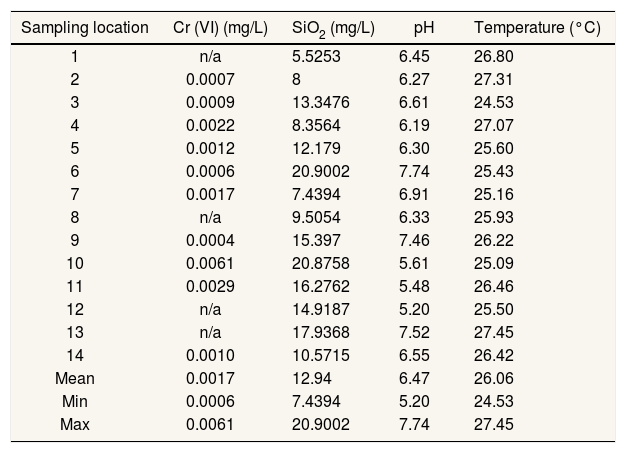
Ecological risk assessmentEcological risk assessment is generally used for the assessment of potential environmental media risks through oral, inhalation, and dermal pathways.13 The pH and concentration of Cr(VI) and SiO2 of drinking water in research area is represented in Table 1. Only 3 stations are specifically at risk for Cr(VI). Nevertheless, heavy metals are toxic and able to accumulate over long periods.14,15
Cr (VI) and SiO2 concentration, pH and temperature.
| Sampling location | Cr (VI) (mg/L) | SiO2 (mg/L) | pH | Temperature (°C) |
|---|---|---|---|---|
| 1 | n/a | 5.5253 | 6.45 | 26.80 |
| 2 | 0.0007 | 8 | 6.27 | 27.31 |
| 3 | 0.0009 | 13.3476 | 6.61 | 24.53 |
| 4 | 0.0022 | 8.3564 | 6.19 | 27.07 |
| 5 | 0.0012 | 12.179 | 6.30 | 25.60 |
| 6 | 0.0006 | 20.9002 | 7.74 | 25.43 |
| 7 | 0.0017 | 7.4394 | 6.91 | 25.16 |
| 8 | n/a | 9.5054 | 6.33 | 25.93 |
| 9 | 0.0004 | 15.397 | 7.46 | 26.22 |
| 10 | 0.0061 | 20.8758 | 5.61 | 25.09 |
| 11 | 0.0029 | 16.2762 | 5.48 | 26.46 |
| 12 | n/a | 14.9187 | 5.20 | 25.50 |
| 13 | n/a | 17.9368 | 7.52 | 27.45 |
| 14 | 0.0010 | 10.5715 | 6.55 | 26.42 |
| Mean | 0.0017 | 12.94 | 6.47 | 26.06 |
| Min | 0.0006 | 7.4394 | 5.20 | 24.53 |
| Max | 0.0061 | 20.9002 | 7.74 | 27.45 |
n/a: not available (below instrument limitation).
Source: Primary Data, 2020.
The maximum WHO contamination level for total chromium in drinking water is 50μg/L. The safe concentration for drinking water consumption of Cr total chromium in Canada is 0.1mg/L.10 Based on Duckham’s research, Cr(VI) minimum risk level is 0.07μg/L. This value based on increased risk to one per million of the average person affected by cancer as a result of drinking well water with Cr(VI) for approximately 70 years.16,17 NOAEL or Screening Benchmark values for Cr(VI) in this study were taken from the CPHGCDW which collected levels of 0.002mg/L.14 The level of health protection taken through toxicity experiment in female mice in the NTP study.18
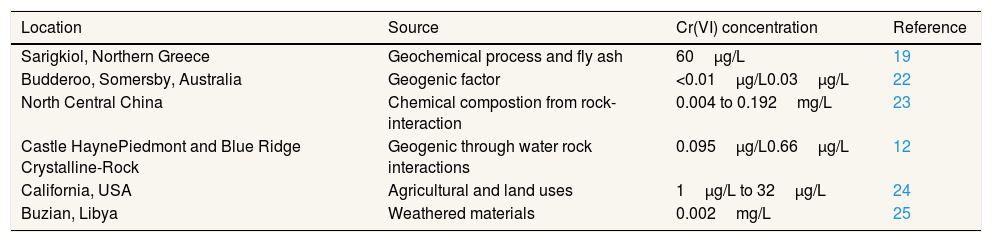
Heavy metals can pollute wells through the movement of groundwater and surface water.15 Leaching processes from topsoil and rock soils are natural sources and play an important role in the flow of chromium to groundwater. The cement industry produces heavy metal dust, gas and particulate matter.4 Research in cement factories in Calabar, consist of the chromium and cadmium content of all water samples (surface water, wells, boreholes, and streams) which found that Cr(VI) concentrations far above WHO recommendations in the region.17 Generally, Cr(VI) concentration in drinking water is about 0.01 to 0.05mg/L and could be more higher in region with deposited of fly ash.19Table 2 shows some concentration data, with different source and area that polluted with Cr(VI) in groundwater.
Concentration and source of Cr(VI) in some research.
| Location | Source | Cr(VI) concentration | Reference |
|---|---|---|---|
| Sarigkiol, Northern Greece | Geochemical process and fly ash | 60μg/L | 19 |
| Budderoo, Somersby, Australia | Geogenic factor | <0.01μg/L0.03μg/L | 22 |
| North Central China | Chemical compostion from rock-interaction | 0.004 to 0.192mg/L | 23 |
| Castle HaynePiedmont and Blue Ridge Crystalline-Rock | Geogenic through water rock interactions | 0.095μg/L0.66μg/L | 12 |
| California, USA | Agricultural and land uses | 1μg/L to 32μg/L | 24 |
| Buzian, Libya | Weathered materials | 0.002mg/L | 25 |
Cr(VI) can be produced naturally in soil and sediment. Solid reactions with solubility and period time are enough to determine the concentration of Cr(VI) in groundwater.20 Cr(VI) and other heavy metals that found around the industrial area is strongly associated with anthropogenic contamination.21
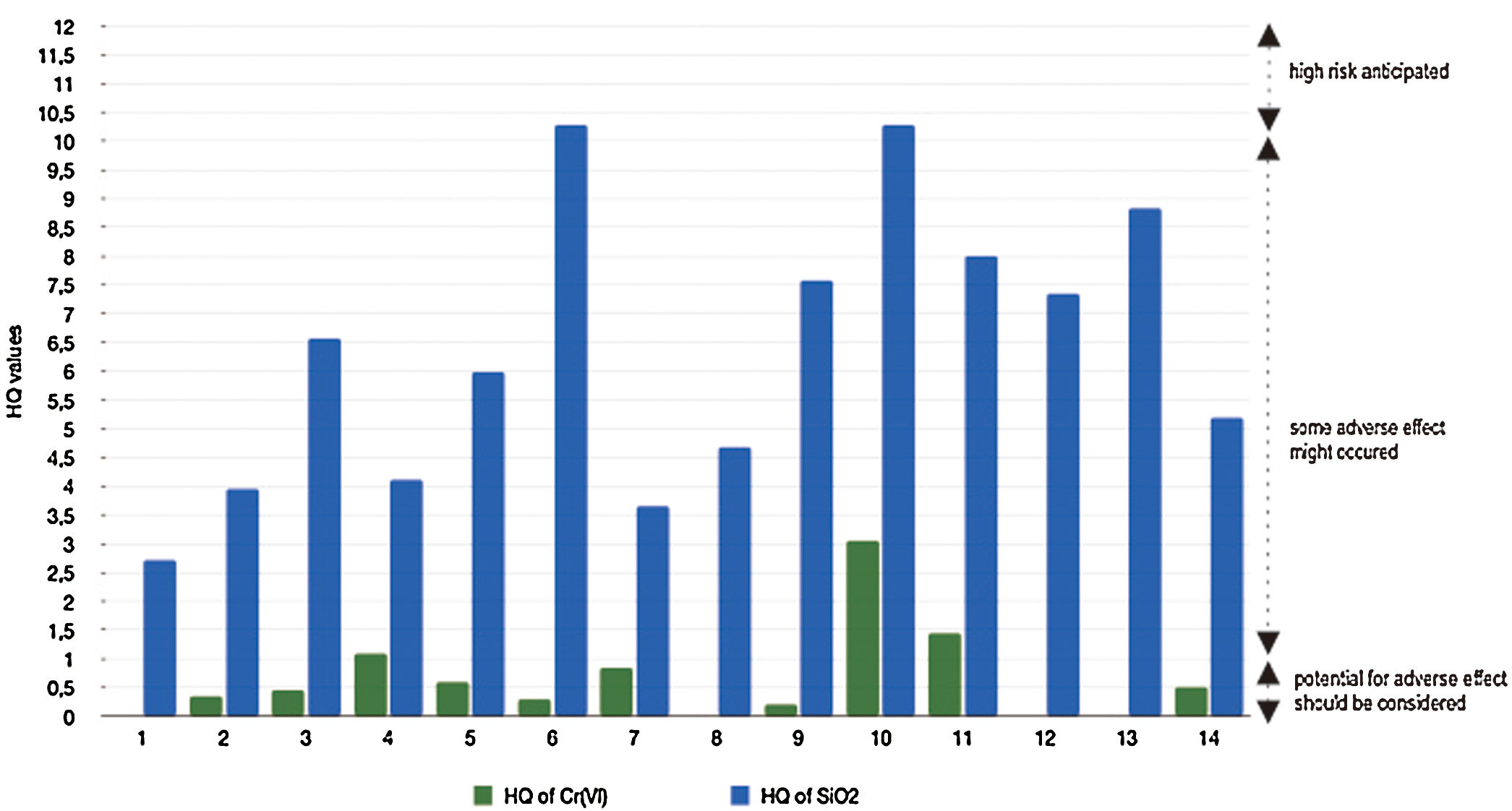
There are various data of genotoxicity and mutagenicity of Cr(VI) in vivo and in vitro. In specific, chromium in its hexavalent form is considered to be a carcinogen.26 Based on Fig. 3, three stations with potential exposure of Cr(VI) through the consumption of well water containing Cr(VI) are station 10 (HQ=3.05), station 11 (HQ=1.45), and station 4 (HQ=1.1). Whereas in the other stations, the HQ value is still below 1 (low risk).
Silicon dioxide does not react directly with water because of its complex covalent structure. In high temperature and concentrated solution, silicon dioxide possibly reacts with sodium hydroxide.27 A feasible positive correlation was found for SiO2 which showed that more silicon dioxide was dissolved by high-temperature water. The risk of well water consumption around the cement industry and karst mines should be limited to reduce human health effects in the future. The quantity of silicon dioxide in well water varies from 1 to 30mg/L.28
Silicon dioxide is one of the compounds that commonly found on Earth's surface. Land expansion will reduce environmental quality due to the entry of silicon dioxide and other toxic minerals into groundwater which is connected to all water sources from karst mountains and resident wells. This process will poses a health hazard to the surrounding population. Data of MRL of silicon dioxide through oral from male rat experiments is 2.030mg/day.29 This is the minimum dose where gastrointestinal problems, hematology and lethality are present. From Fig. 3, it can be seen that SiO2 has higher HQ values than Cr(VI). All stations have HQ>1. This indicates that mostly areas have a moderate level of ecological risk. Only two stations with HQ value are more than 10, both of them located in Tukamasea Village.
The pH trends throughout the station are in neutral (slightly acid) conditions. Other mineral contents may affect the composition of hydrogen ions so the obtained pH is less acid due to the rock weathering process.30 In a region with the highest Cr(VI) content, pH 5.52 was recorded. In contrast, the station with the highest silicon dioxide concentration was slightly alkali (pH 7.74). The possible way to reducing Cr(VI) and SiO2 pollutants in environment are reductions, coagulation, and filtration process. Low base ion exchange and strong base ion exchange are effective technologies for removing Cr(VI) from drinking water. The risk of water consumption of wells around the cement industry and karst mines should be limited to reduce acute and carcinogenic effects in the future.
ConclusionsThe HQ was calculated to assess the well water quality of 14 samples from Maros Karst area, Indonesia, and health risks caused by exposure to Cr(VI), and silicon dioxide were also considered. Understand and aware contaminants origin from groundwater through the leaching, geological and anthropogenic processes are necessary. The obtained HQ values shall be considered as an evidence base and provide valuable information to identify the source of chemical species. This research is really important in managing and preserving valuable groundwater resources.
Conflicts of interestsThe authors declare that they have no conflict of interest.
Peer-review under responsibility of the scientific committee of the 1st International Conference on Safety and Public Health (ICOS-PH 2020). Full-text and the content of it is under responsibility of authors of the article.