To evaluate the risk of acute pancreatitis and biliary disease in patients treated with glucagon-like peptide-1 receptor agonists (GLP-1 RA).
MethodPopulation-based, propensity-weighted, new user, active comparator design study including patients with diabetes and obesity initiating treatment with GLP-1 RA or the comparator group sodium-glucose cotransporter 2 inhibitors (SGLT-2i) in the region of Valencia from 2015 to 2021.
ResultsIn adjusted, per protocol main analysis, no risk differences were found for acute pancreatitis (HR: 0.56; 95%CI: 0.17-1.91) nor for biliary disease (HR: 1.12; IC95%: 0.79, 1.58). Secondary analyses yielded similar results.
ConclusionsDespite we did not observe increased risk of gastrointestinal events in GLP-1 RA vs SGLT-2i patients, adherence to approved indications and close monitoring of potential adverse events are warranted to ensure patient safety.
Evaluar el riesgo de pancreatitis aguda y enfermedad biliar en pacientes tratados con agonistas del receptor del péptido similar al glucagón tipo 1 (aGLP-1).
MétodoEstudio de base poblacional, ajustado por índices de propensión, de nuevos usuarios y con comparador activo, incluyendo pacientes con diabetes y obesidad que iniciaron tratamiento con aGLP-1 o con un comparador inhibidor del cotransportador de sodio-glucosa 2 (iSGLT-2) en la Comunidad Valenciana, desde 2015 hasta 2021.
ResultadosEn el análisis principal, ajustado por protocolo, no se encontraron diferencias en el riesgo de pancreatitis aguda (HR: 0,56; IC95%: 0,17-1,91) y de enfermedad biliar (HR: 1,12; IC95%: 0,79-158). Los análisis secundarios arrojaron resultados similares.
ConclusionesA pesar de que no se observó un mayor riesgo de eventos gastrointestinales en los pacientes con aGLP-1 frente a los tratados con iSGLT-2, es necesario asegurar el cumplimiento de las indicaciones aprobadas, así como una estrecha monitorización de posibles eventos adversos para garantizar la seguridad de los pacientes.
In the last years, the popularity of glucagon-like peptide 1 receptor agonists (GLP-1 RA), a drug class traditionally employed to treat type 2 diabetes, has grown exponentially, mostly around their use for weight loss, after the authorisation of some GLP-1RA as weight loss agents, and fuelled by media and celebrities attention. GLP-1 RA use has been linked to potentially severe gastrointestinal adverse effects such as pancreatitis, bowel obstruction or biliary disease, but evidence is based on studies that either are not adequately designed to capture those events or to infer causality,1–3 or in observational studies with a debatable choice of comparator groups.4,5 We evaluated the association between GLP-1 RA use and increased risk for acute pancreatitis and biliary disease using population-based real-world data from a Spanish region with 5 million inhabitants.
MethodDesign, setting, and data sourcesReal-world, active comparator, new user retrospective cohort study combining several population-wide databases from the Valencia Health System Integrated Database (VID). VID is a set of multiple, public, population-wide electronic databases for the Valencia Region, the fourth most populated Spanish region, with approximately 5 million inhabitants. VID provides exhaustive longitudinal information including sociodemographic and administrative data, clinical, pharmaceutical prescription and dispensing linked at the individual level (including brand and generic name, formulation, strength and dosing schedule/regimen), and healthcare utilization data from public hospital care, emergency departments, specialized care, primary care and other public health services.6
We included new users of GLP-1 RA agonists and an active comparator, sodium-glucose cotransporter 2 inhibitors (SGLT-2i), in the period Jan 1, 2015 to December 31, 2021 (see Table S1 in online Supplementary Material for Anatomical Therapeutic Chemical Classification of drugs included). Patients receiving a prescription for either GLP-1 RA or SGLT-2i medication and not having received a prescription for an antidiabetic drug (except for metformin) in the previous 365 days were considered new users. Considering that SGLT-2i and GLP-1RA are recommended as therapeutic alternatives by many clinical guidelines, usually as second-line treatments after first-line metformin or other non-insulin treatment, new users of SGLT-2i appear to be a suitable active comparator group. The index date was the day of first prescription of a study drug. In Spain GLP-1RA for diabetes mellitus are only authorised for individuals with obesity. Accordingly, we only included new SGLT-2i or GLP-1RA users with body mass index (BMI) ≥30 when initiating therapy (see operational definition in Table 1, footnote), in order to produce groups as similar as possible at baseline. Patients in both groups were required to use metformin in the index date (defined by having a prescription for metformin issued in the 60 days before the index date).
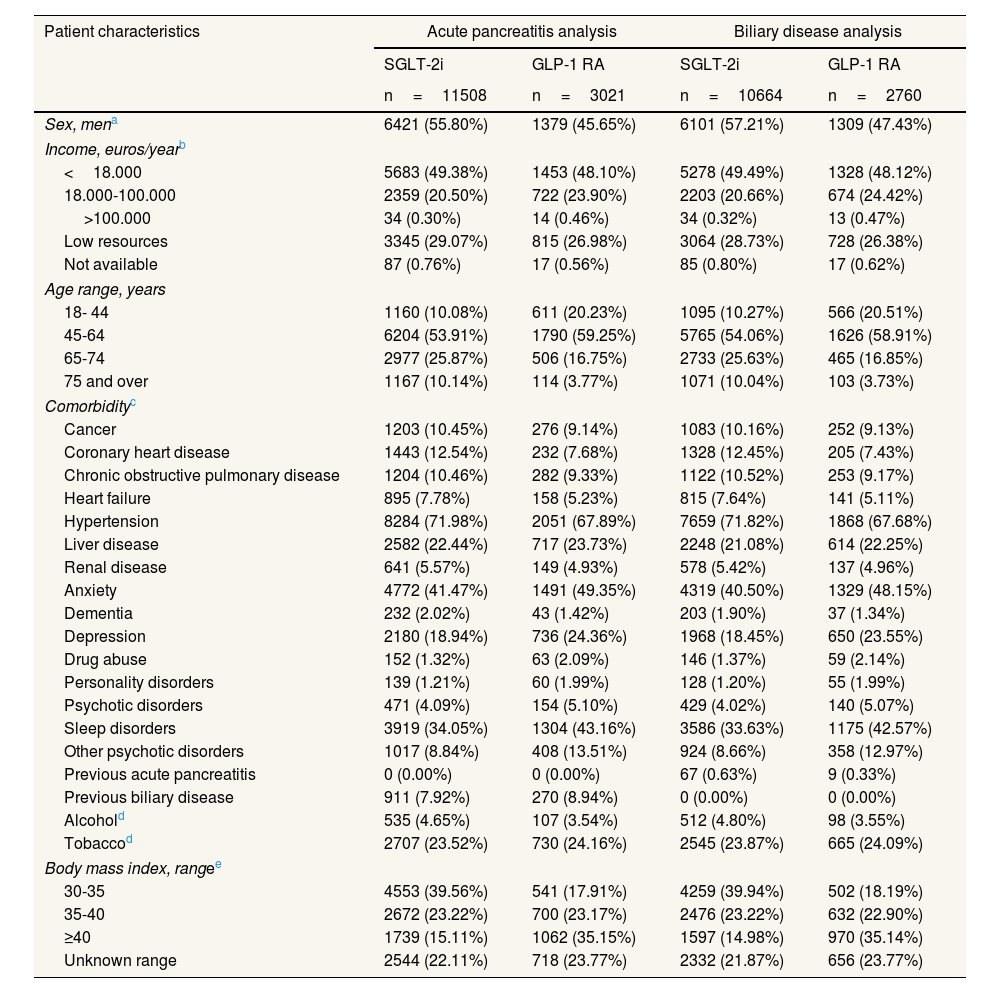
Patient characteristics for the acute pancreatitis and the biliary disease analyses for patients initiating GLP-1 RA or SGLT-2i.
| Patient characteristics | Acute pancreatitis analysis | Biliary disease analysis | ||
|---|---|---|---|---|
| SGLT-2i | GLP-1 RA | SGLT-2i | GLP-1 RA | |
| n=11508 | n=3021 | n=10664 | n=2760 | |
| Sex, mena | 6421 (55.80%) | 1379 (45.65%) | 6101 (57.21%) | 1309 (47.43%) |
| Income, euros/yearb | ||||
| <18.000 | 5683 (49.38%) | 1453 (48.10%) | 5278 (49.49%) | 1328 (48.12%) |
| 18.000-100.000 | 2359 (20.50%) | 722 (23.90%) | 2203 (20.66%) | 674 (24.42%) |
| >100.000 | 34 (0.30%) | 14 (0.46%) | 34 (0.32%) | 13 (0.47%) |
| Low resources | 3345 (29.07%) | 815 (26.98%) | 3064 (28.73%) | 728 (26.38%) |
| Not available | 87 (0.76%) | 17 (0.56%) | 85 (0.80%) | 17 (0.62%) |
| Age range, years | ||||
| 18- 44 | 1160 (10.08%) | 611 (20.23%) | 1095 (10.27%) | 566 (20.51%) |
| 45-64 | 6204 (53.91%) | 1790 (59.25%) | 5765 (54.06%) | 1626 (58.91%) |
| 65-74 | 2977 (25.87%) | 506 (16.75%) | 2733 (25.63%) | 465 (16.85%) |
| 75 and over | 1167 (10.14%) | 114 (3.77%) | 1071 (10.04%) | 103 (3.73%) |
| Comorbidityc | ||||
| Cancer | 1203 (10.45%) | 276 (9.14%) | 1083 (10.16%) | 252 (9.13%) |
| Coronary heart disease | 1443 (12.54%) | 232 (7.68%) | 1328 (12.45%) | 205 (7.43%) |
| Chronic obstructive pulmonary disease | 1204 (10.46%) | 282 (9.33%) | 1122 (10.52%) | 253 (9.17%) |
| Heart failure | 895 (7.78%) | 158 (5.23%) | 815 (7.64%) | 141 (5.11%) |
| Hypertension | 8284 (71.98%) | 2051 (67.89%) | 7659 (71.82%) | 1868 (67.68%) |
| Liver disease | 2582 (22.44%) | 717 (23.73%) | 2248 (21.08%) | 614 (22.25%) |
| Renal disease | 641 (5.57%) | 149 (4.93%) | 578 (5.42%) | 137 (4.96%) |
| Anxiety | 4772 (41.47%) | 1491 (49.35%) | 4319 (40.50%) | 1329 (48.15%) |
| Dementia | 232 (2.02%) | 43 (1.42%) | 203 (1.90%) | 37 (1.34%) |
| Depression | 2180 (18.94%) | 736 (24.36%) | 1968 (18.45%) | 650 (23.55%) |
| Drug abuse | 152 (1.32%) | 63 (2.09%) | 146 (1.37%) | 59 (2.14%) |
| Personality disorders | 139 (1.21%) | 60 (1.99%) | 128 (1.20%) | 55 (1.99%) |
| Psychotic disorders | 471 (4.09%) | 154 (5.10%) | 429 (4.02%) | 140 (5.07%) |
| Sleep disorders | 3919 (34.05%) | 1304 (43.16%) | 3586 (33.63%) | 1175 (42.57%) |
| Other psychotic disorders | 1017 (8.84%) | 408 (13.51%) | 924 (8.66%) | 358 (12.97%) |
| Previous acute pancreatitis | 0 (0.00%) | 0 (0.00%) | 67 (0.63%) | 9 (0.33%) |
| Previous biliary disease | 911 (7.92%) | 270 (8.94%) | 0 (0.00%) | 0 (0.00%) |
| Alcohold | 535 (4.65%) | 107 (3.54%) | 512 (4.80%) | 98 (3.55%) |
| Tobaccod | 2707 (23.52%) | 730 (24.16%) | 2545 (23.87%) | 665 (24.09%) |
| Body mass index, rangee | ||||
| 30-35 | 4553 (39.56%) | 541 (17.91%) | 4259 (39.94%) | 502 (18.19%) |
| 35-40 | 2672 (23.22%) | 700 (23.17%) | 2476 (23.22%) | 632 (22.90%) |
| ≥40 | 1739 (15.11%) | 1062 (35.15%) | 1597 (14.98%) | 970 (35.14%) |
| Unknown range | 2544 (22.11%) | 718 (23.77%) | 2332 (21.87%) | 656 (23.77%) |
Income level: retrieved from the pharmaceutical copayment system that establishes different levels of copayment based on income categories, population exempt from copayment due to socioeconomic vulnerability (labelled in our study as the ‘low resources’ group), and people without income data (labelled as ‘not available’).
Comorbidity: active comorbidity in VID at baseline (diagnostic codes in the VID electronic medical record may be activated at the onset of a disease and deactivated after; typically diagnostic codes for chronic conditions remain active for life).
Alcohol and tobacco: registered tobacco and alcohol use in VID in the 365 days preceding the index date.
Body mass index at baseline, defined as a measure ≥30, or a diagnose of obesity using International Classification of Diseases, Ninth and Tenth Revision (ICD-9 and ICD-10), registered in the VID medical record in the 365 days before the index date. Unknown range: patients classified as obese via ICD code with no strata specification.
We included the following patient sociodemographic, lifestyle and clinical variables at the individual-level: age, sex assigned at birth, income level, comorbidities at baseline (heart failure, dementia, hypertension, liver disease, renal disease, coronary heart disease, congestive obstructive pulmonary disease, malignancies, depression, sleep disorders, anxiety, substance abuse, personality disorders, psychotic disorders, other psychiatric disorders), BMI strata at baseline, previous acute pancreatitis and previous biliary disease (see Table 1 and Table S2 in online Supplementary Material for ICD codes employed).
OutcomesPrimary outcomes were acute pancreatitis (ICD code: 577; ICD-10 codes: K85, K86) and biliary disease, including cholelithiasis (ICD-9 codes; 574-575-576; ICD-10 codes: K80-K81-K82-K83). Outcomes were retrieved from different real-world databases in VID (primary care electronic records, in-hospital and outpatient hospital records and emergency room records), via ICD-10 codes registered during healthcare encounters. In the main per-protocol analyses, participants were followed from the index date to first mutually exclusive incidence of the outcome of interest, discontinuation of study drug (defined as more than 60 days without drug supply; days of drug supply was estimated using dosing information and dispensation data), initiation of other study drug, or switch to other glucose-lowering drug (defined as receiving a prescription for any glucose-lowering drug other than the baseline treatment or metformin and not refilling the assigned drug for 60 days), death, or end of follow-up. In secondary, intention-to-treat analyses, participants were followed from the index date to first event, death, or end of follow-up (Dec 31, 2021), and were analysed according to the treatment assigned at baseline, irrespective of actual exposure during follow-up.
AnalysisFirst, we described patient characteristics of GLP-1RA and SGLT-2i users included in the main analysis of acute pancreatitis and of biliary disease. For each analysis, new users that had no previous event of interest registered in VID before the index date were included. Second, as main analyses, we used a per-protocol approach to estimate the risk of pancreatitis and biliary disease in GLP-1RA vs. SGLT-2i. We carried out multivariable Cox regression modelling using our set of covariates. To adjust for potential confounding, we used inverse probability of treatment weighting based on propensity scores (PS). PS for each outcome were calculated based on the probability of initiating treatment with GLP-1RA taking into account the same covariates, to generate patient-specific stabilised weights. Covariate balance between the weighted exposure cohorts was assessed using standardised mean differences, with standardised differences <0.10 suggesting adequate balance.7 As secondary analyses, we provided estimates for patients with a previous outcome of interest before the index date, as well as intention-to-treat analyses. Statistical significance was defined as 0.05. Analyses were performed using STATA version 14 and R version 3.6.0. Ethics approval was obtained from the Valencia Hospital Clinic Universitari's clinical research ethics board (2022/163) with a waiver of informed consent.
ResultsWe included 11,508 new users of SGLT-2i and 3021 new users of GLP-1 RA in the acute pancreatitis analysis, and 10,664 and 2760 new users in the biliary disease analysis, respectively (Table 1). Mean follow-up was 482.9 days for GLP-1 RA users and 397.1 days for SGLT-2i users in per protocol analyses, and 991.8 and 969.7 in intention-to-treat analyses, respectively. Standardized mean differences in covariates between groups after PS weighting showed adequate balance after adjustment for main analyses (see Table S3 in online Supplementary Material). In adjusted, per protocol, main analyses, no risk differences were found for acute pancreatitis (hazard ratio [HR]: 0.56; 95% confidence interval [95CI%]: 0.17-1.91) nor for biliary disease (HR: 1.12; 95%IC: 0.79-1.58) (Table 2). Intention-to-treat analyses and analyses in patients with previous outcomes (including 119 and 19 patients in the SGLT-2i and GLP1 RA groups, respectively, in the pancreatitis analysis, and 963 and 280 patients, respectively, in the biliary disease analysis) also yielded non-significant results (Table 2).
Risk of acute pancreatitis and biliary disease among new users of GLP-1RA vs SGLT-2i, without and with previous event.
| Outcome | Analysis | Group | Person-time | Weighted events | Weighted rate/1000 person-year | 95%CI | HR | 95%CI |
|---|---|---|---|---|---|---|---|---|
| Patients without previous event | ||||||||
| Acute pancreatitis | Per protocol analysis | SGLT-2i | 12457.00 | 23.46 | 1.88 | 1.27-2.91 | Ref. | |
| GLP-1 RA | 3760.15 | 3.94 | 1.05 | 0.33-5.13 | 0.56 | 0.17-1.91 | ||
| Intention to treat analysis | SGLT-2i | 31452.40 | 66.03 | 2.10 | 1.65-2.71 | Ref. | ||
| GLP-1 RA | 7526.65 | 17.65 | 2.34 | 1.38-4.33 | 1.11 | 0.61-2.03 | ||
| Biliary disease | Per protocol analysis | SGLT-2i | 11388.36 | 138.59 | 12.17 | 10.29-14.51 | Ref. | |
| GLP-1 RA | 3333.75 | 46.84 | 14.05 | 10.57-19.08 | 1.12 | 0.79-1.58 | ||
| Intention to treat analysis | SGLT-2i | 28657.17 | 347.88 | 12.14 | 10.90-13.56 | Ref. | ||
| GLP-1 RA | 6674.24 | 91.75 | 13.75 | 11.21-17.03 | 1.11 | 0.88-1.41 | ||
| Patients with previous event | ||||||||
| Acute pancreatitis | Per protocol analysis | SGLT-2i | 91.35 | 29.10 | 318.49 | 200.45-510.99 | Ref. | |
| GLP-1 RA | 16.25 | 3.47 | 213.37 | 46.25-933.47 | 0.67 | 0.18-2.56 | ||
| Intention to treat analysis | SGLT-2i | 194.35 | 38.29 | 197.02 | 134.2-291.8 | Ref. | ||
| GLP-1 RA | 26.86 | 5.06 | 188.18 | 50.35-699.58 | 0.96 | 0.31-2.92 | ||
| Biliary disease | Per protocol analysis | SGLT-2i | 871.60 | 124.12 | 142.40 | 117.54-173.49 | Ref. | |
| GLP-1 RA | 333.35 | 49.39 | 148.15 | 100.24-221.99 | 1.20 | 0.82-1.77 | ||
| Intention to treat analysis | SGLT-2i | 2030.69 | 213.23 | 105.00 | 90.60-122.08 | Ref. | ||
| GLP-1 RA | 561.98 | 67.57 | 120.23 | 85.67-171.45 | 1.15 | 0.82-1.61 | ||
95%CI: 95% confidence interval; HR: hazard ratio.
In this propensity-weighted, new user, active comparator study, we did not observe increased risk of acute pancreatitis nor biliary complications in patients with diabetes and obesity initiating GLP-1 RA when compared to SGLT-2i initiators. We evaluated patients with and without previous acute pancreatitis and biliary disease, and we provided per protocol and intention-to-treat estimates, consistently finding no association between GLP1 RA and increased risk. Our results do not confirm the higher risk observed in other observational studies. However, these studies do not control for important confounders such as BMI, and comparator groups differ significantly at baseline from GLP-1 RA patients.4,5 A systematic review including 76 randomized clinical trials found increased risk of gallbladder and biliary disease associated with GLP-1 RA; however, no significant increase was observed when including only active-comparator trials.8 A potential effect of both comparator and GLP-1 RA groups in these studies (and ours) cannot therefore be ruled out.
Our study is subject to some limitations: first, real-world, daily practice observational data may be affected by many potential biases; second, despite inverse probability of treatment weighting adjustment, residual confounding is possible; third, small number of events in the acute pancreatitis analysis result in potentially imprecise estimates, a cautious interpretation is warranted; fourth, we did not include private care data, even if it is marginal in our setting; fifth, we cannot discard a potential effect of both drugs on the outcomes; sixth, even if it is highly unlikely, there is a potential use of GLP1 RA in non-diabetic patients; and finally, extrapolation to other settings should be approached with prudence. Use of GLP-1 RA is growing exponentially. Despite we did not observe an increase in gastrointestinal events associated with this drug class when compared to SGLT-2i in patients with diabetes type 2 and obesity, we cannot fully discard a risk increase. Adherence to approved indications and close monitoring of potential adverse events and of patients at a potentially higher risk is warranted to ensure a safe use of GLP-1 RA.
Availability of databases and material for replicationData are available upon reasonable request. The datasets presented in this article are not readily available due to legal restrictions on sharing the data set as regulated by the Valencia regional government by means of legal resolution by the Valencia Health Agency which forbids the dissemination of data to third parties (accessible at: https://www.san.gva.es/es/web/investigacio/introduccion). Upon request, authors can allow access to the databases to verify the accuracy of the analysis or the reproducibility of the study. Requests to access the datasets should be directed to Management Office of the Data Commission in the Valencia Health Agency.
Editor in chargePilar Pinilla Domínguez.
A few studies have observed higher risk of gastrointestinal events such as acute pancreatitis or biliary diseases in patients taking glucagon-like peptide-1 receptor agonists (GLP-1 RA). However, evidence is scarce and subject to many limitations.
What does this study add to the literature?In this propensity-weighted, new user, active comparator design study, we did not observe increased risk of acute pancreatitis nor biliary disease in patients with diabetes and obesity initiating GLP-1 RA when compared to those initiating sodium-glucose cotransporter 2 inhibitors (SGLT-2i) between 2015 and 2021 in the region of Valencia, Spain.
What are the implications of the results?Despite we did not observe an increase in gastrointestinal events associated with GLP-1 RA when compared to SGLT-2i, we cannot fully discard a potential effect, and adherence to approved indications and close patient monitoring is warranted to ensure a safe use of GLP-1 RA.
The corresponding author, on behalf of the other authors guarantee the accuracy, transparency and honesty of the data and information contained in the study, that no relevant information has been omitted and that all discrepancies between authors have been adequately resolved and described.
Authorship contributionsG. Sanfélix-Gimeno had full access to all the study data and assumes responsibility for the integrity of the data and the precision of the analysis. Concept and design: C. Robles Cabanillas, I. Hurtado Navarro, G. Sanfélix-Gimeno and A. García-Sempere. Data acquisition, analysis, and interpretation: all authors. Writing of the text: C. Robles Cabanillas and A. García-Sempere. Critical review of the text and important intellectual contribution: all authors. Statistical analysis: C. Robles Cabanillas and I. Hurtado Navarro. Supervision: G. Sanfélix-Gimeno, I. Hurtado Navarro and A. García-Sempere.
FundingThis research was co-funded by RD21/0016/0006 of the Carlos III Health Institute (ISCIII), Ministry of Science, Innovation and Universities, and by the Recovery, Transformation and Resilience Plan through NextGenerationEU European funds.
Conflicts of interestAll authors are employees of FISABIO, a research organization of in Spain affiliated with the Department of Health of the Generalitat Valenciana. FISABIO signed a collaboration agreement (2021-25) with RTI International to conduct research on management patterns and incidence of cardiovascular and renal outcomes in patients with diabetes and kidney disease, unrelated with the present work.