To investigate the causal relationship between poor lifestyle habits, such as smoking and drinking, and cutaneous malignant melanoma.
MethodIn the present study, alcohol consumption and smoking were used as exposure factors, and single nucleotide polymorphisms closely associated with alcohol consumption and smoking were used as instrumental variables, while cutaneous melanoma was set as an outcome variable. Two-sample Mendelian randomization analyses were run between alcohol consumption and melanoma and smoking and melanoma to investigate their causal associations, respectively.
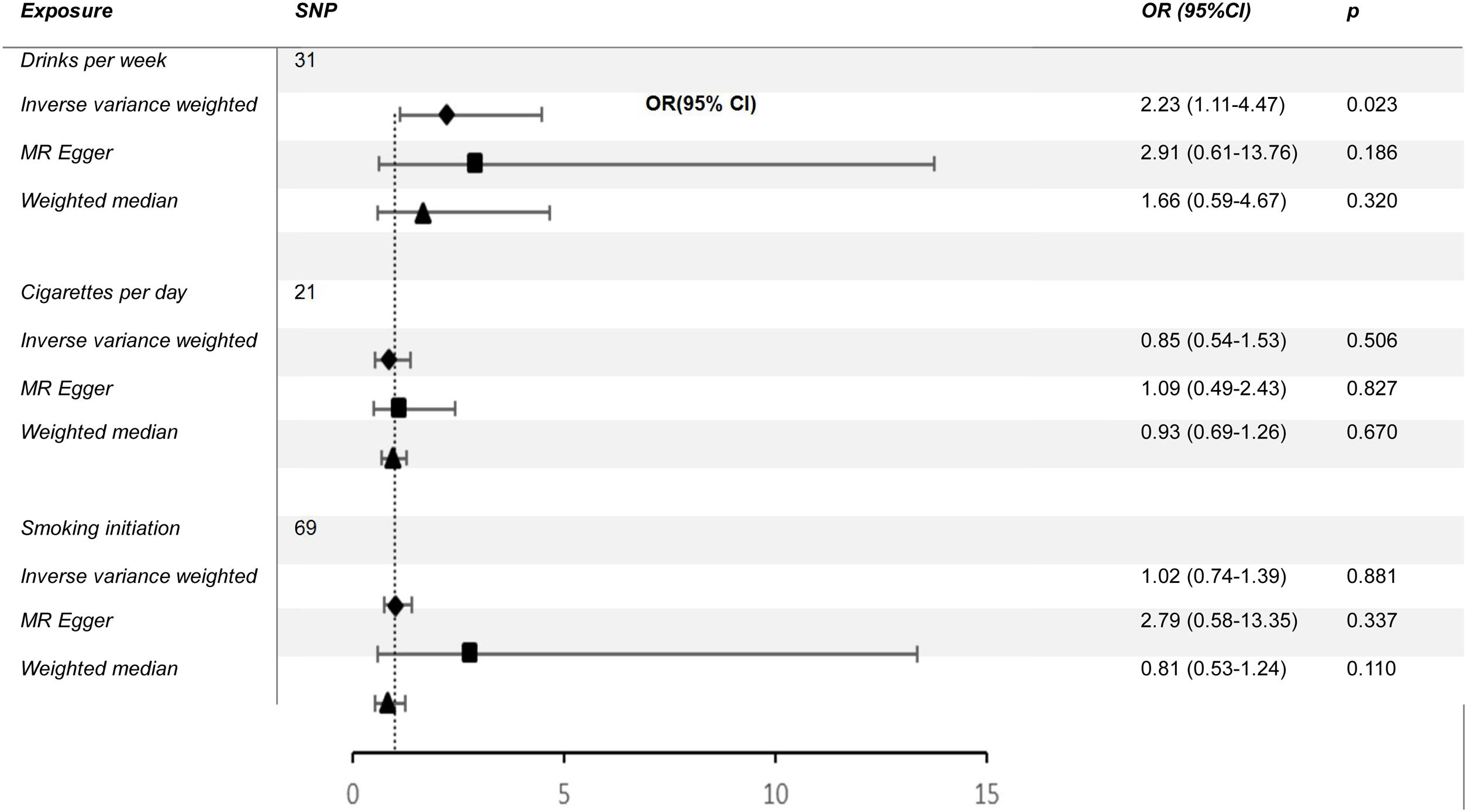
ResultsWe found a positive and statistically significant causal effect of alcohol intake on the risk of cutaneous malignant melanoma (OR: 2.23; 95%CI: 1.11-4.47; p=0.02). The present study showed no significant causal relationship between cigarettes per day and cutaneous melanoma (OR: 0.85; 95%CI: 0.54-1.35; p=0.50) or smoking initiation and cutaneous melanoma (OR: 1.02; 95%CI: 0.74-1.39; p=0.88).
ConclusionsThis study provides Mendelian randomization evidence supporting alcohol consumption as a risk factor for cutaneous malignant melanoma. And the causal relationship between smoking and cutaneous malignant melanoma still needs to be further investigated.
Investigar la relación causal entre los malos hábitos de vida, como el tabaquismo y el consumo de alcohol, y el melanoma maligno cutáneo.
MétodoEn el presente estudio, el consumo de alcohol y el tabaquismo se utilizaron como factores de exposición, y los polimorfismos de nucleótido único estrechamente asociados con el consumo de alcohol y el tabaquismo se utilizaron como variables instrumentales, mientras que el melanoma cutáneo se estableció como variable de resultado. Se realizaron análisis de aleatorización mendeliana de dos muestras entre el consumo de alcohol y el melanoma, y entre el tabaquismo y el melanoma, para investigar sus asociaciones causales, respectivamente.
ResultadosSe encontró un efecto causal positivo y estadísticamente significativo del consumo de alcohol sobre el riesgo de melanoma maligno cutáneo (OR: 2,23; IC95%: 1,11-4,47; p=0,02). El presente estudio no mostró una relación causal significativa entre cigarrillos por día y melanoma cutáneo (OR: 0,85; IC95%: 0,54-1,35; p=0,50) ni entre inicio de fumar y melanoma cutáneo (OR: 1,02; IC95%: 0,74-1,39; p=0,88).
ConclusionesEste estudio aporta pruebas de aleatorización mendeliana que apoyan el consumo de alcohol como factor de riesgo de melanoma maligno cutáneo. En cuanto a la relación causal entre el tabaquismo y el melanoma maligno cutáneo aún debe investigarse más a fondo.
Cutaneous melanoma is a malignant tumor caused by the uncontrolled growth of melanocytes in the skin that become cancerous. Skin cancer can be divided into melanoma skin cancer and non-melanoma skin cancer, of which melanoma accounts for far less than the latter, but its mortality rate accounts for 73% of the total mortality rate of skin cancer, which demonstrates its dangerousness. Early diagnosis of melanoma is critical because the 5-year survival rate for patients treated with surgical resection in early-stage melanoma (approximately 98%) is much higher than in advanced or metastatic stages. For the general public, sudden onset of dark brown patches on the skin or sudden darkening, enlargement, rupture, pain, and itching of a stable pigmented mole are important signs of melanoma. In addition, the A (asymmetry), B (border irregularity), C (color variation), D (diameter>6mm) and E (evolving) principle proposed by the American Academy of Dermatologists is an important guideline for effective detection of melanoma.
In addition to risk factors such as ultraviolet radiation, trauma, and repeated friction, in recent years, numerous papers have pointed out that drinking and smoking are also associated with an increased incidence of melanoma. In an article with a meta-analysis on alcohol consumption and melanoma, a moderate association between alcohol consumption and increased risk of melanoma was noted.1 It has also been shown that ethanol can block the developmental process of melanocytes and directly contribute to the disease progression of melanoma.2 As for smoking and melanoma, studies have shown that smoking is negatively associated with melanoma incidence3 and may even reduce its mortality rate.4 Nevertheless, previous studies do not exclude the influence of confounding factors and there may be bias. The causal relationship between alcohol consumption, smoking, and skin melanoma development remains undetermined.
In the genetic process studied by Mendelian randomization (MR), the assignment of genetically controlled traits follows a strict randomization principle, which is similar to a randomized controlled experiment, which radically reduces the influence of confounding factors. Since the linkage between alleles and disease is invariant and does not change with disease progression, reverse causality can be greatly reduced.
This study used the published Genome-Wide Association Study (GWAS) pooled data on the association between alcohol consumption and smoking and genetic variation in melanoma to estimate the genetic association between exposure and disease, which has tremendous potential clinical and public health implications.
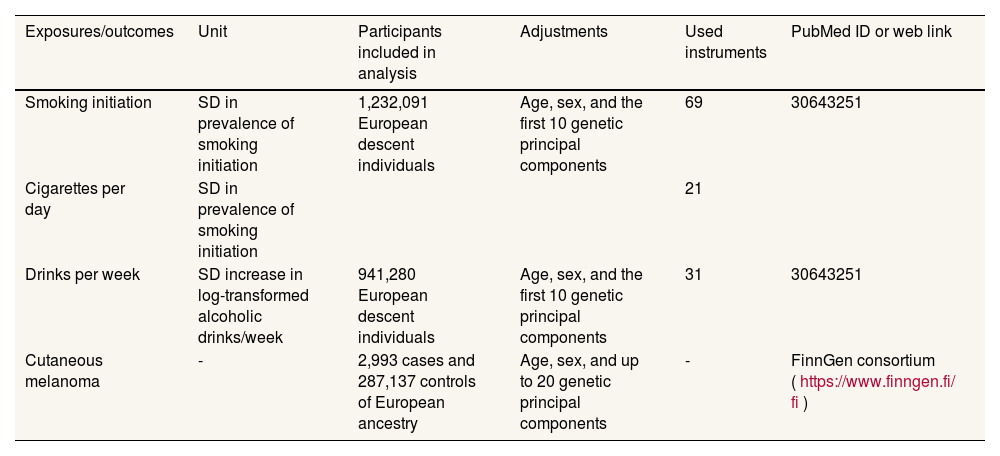
MethodIn the present study, alcohol consumption and smoking were used as exposure factors, and single nucleotide polymorphisms closely associated with alcohol consumption and smoking were used as instrumental variables, while cutaneous melanoma was set as an outcome variable.5 Two-sample MR analyses were run between alcohol consumption and melanoma and smoking and melanoma to investigate their causal associations, respectively. An overview of the MR analysis process can be found in figure 1, and details of the data sources for exposure and outcome can be found in Table 1.
Information on genetic instruments and outcome data sources.
| Exposures/outcomes | Unit | Participants included in analysis | Adjustments | Used instruments | PubMed ID or web link |
|---|---|---|---|---|---|
| Smoking initiation | SD in prevalence of smoking initiation | 1,232,091 European descent individuals | Age, sex, and the first 10 genetic principal components | 69 | 30643251 |
| Cigarettes per day | SD in prevalence of smoking initiation | 21 | |||
| Drinks per week | SD increase in log-transformed alcoholic drinks/week | 941,280 European descent individuals | Age, sex, and the first 10 genetic principal components | 31 | 30643251 |
| Cutaneous melanoma | - | 2,993 cases and 287,137 controls of European ancestry | Age, sex, and up to 20 genetic principal components | - | FinnGen consortium (https://www.finngen.fi/ fi) |
SD: standard deviation.
This studies have been approved by the institutional ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Genetic variants selection criteriaAt first, the single nucleotide polymorphisms (SNP) associated with smoking can be specifically classified as smoking initiation and daily smoking, and in the meta-analysis of smoking and drinking we obtained 5217 (drinks per week), 2129 (cigarettes per day) and 7846 (smoking initiation) SNP based on a significance threshold of (P <5 × 10-8), respectively. In order to exclude the effect of linkage disequilibrium, we used European population reference in the genome of 1000 individuals as the reference panel, set r2 <0.001, window size <10,000 Kb, and finally obtained 37 (drinks per week), 23 (cigarettes per day) and 93 (smoking initiation) SNP. We then harmonized the exposures and results by removing some SNP due to homozygotes with intermediate allele frequencies, resulting in 31 (drinks per week), 21 (cigarettes per day), and 69 (smoking initiation) SNP, which were used as instrumental variables for smoking and drinking.6
Cutaneous melanoma data sourcesThe source of GWAS summary-level data for cutaneous malignant melanoma (excluding other cancers) was the FinnGen consortium, which recruited 429,209 participants, containing 2993 examples and 287,137 controls. Identification of cutaneous malignant melanoma according to International Classification of Diseases 10th edition (ICD-10), ICD-9, ICD-8, Topography ICD-O-3 and Morphology ICD-O-3 codes, and individuals were removed according to genotype QC, resulting in 3194 individuals (female 1542 and male 1652). Details of the GWAS data can be found in Table 1.
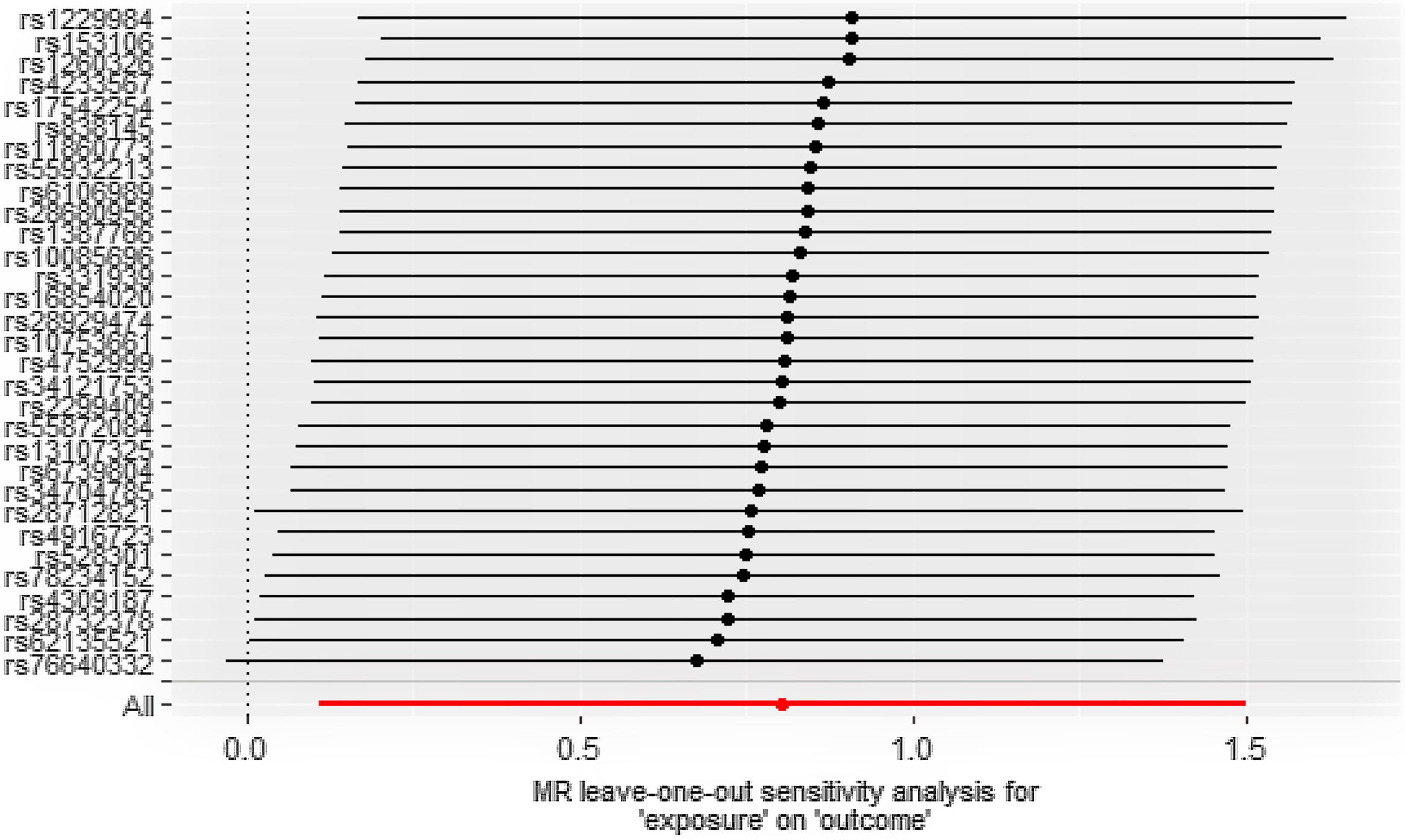
Statistical analysisWe were exploring the potential causal relationship between smoking and alcohol consumption and cutaneous malignant melanoma using inverse (IVW) as the primary analysis method. We also use weighted median and MR-Egger as supplementary analysis methods to IVW, which are more widely used, however, less efficient, and in case of inconsistency between the results and IVW results, we still take the IVW results as the basis.7 We performed heterogeneity analysis of SNPs using the Cochran Q test to determine whether there was bias in the data between samples, and if their p-value was <0.05, the results were not heterogeneous.8 Sensitivity analysis was performed using the leave-one-out method, by deleting single SNP and calculating the effect values of the remaining SNP to determine if they were outliers.9 For horizontal pleiotropy, we used the Egger-intercept method of testing to determine whether exposure affects outcome through other pathways with the help of its intercept term.10
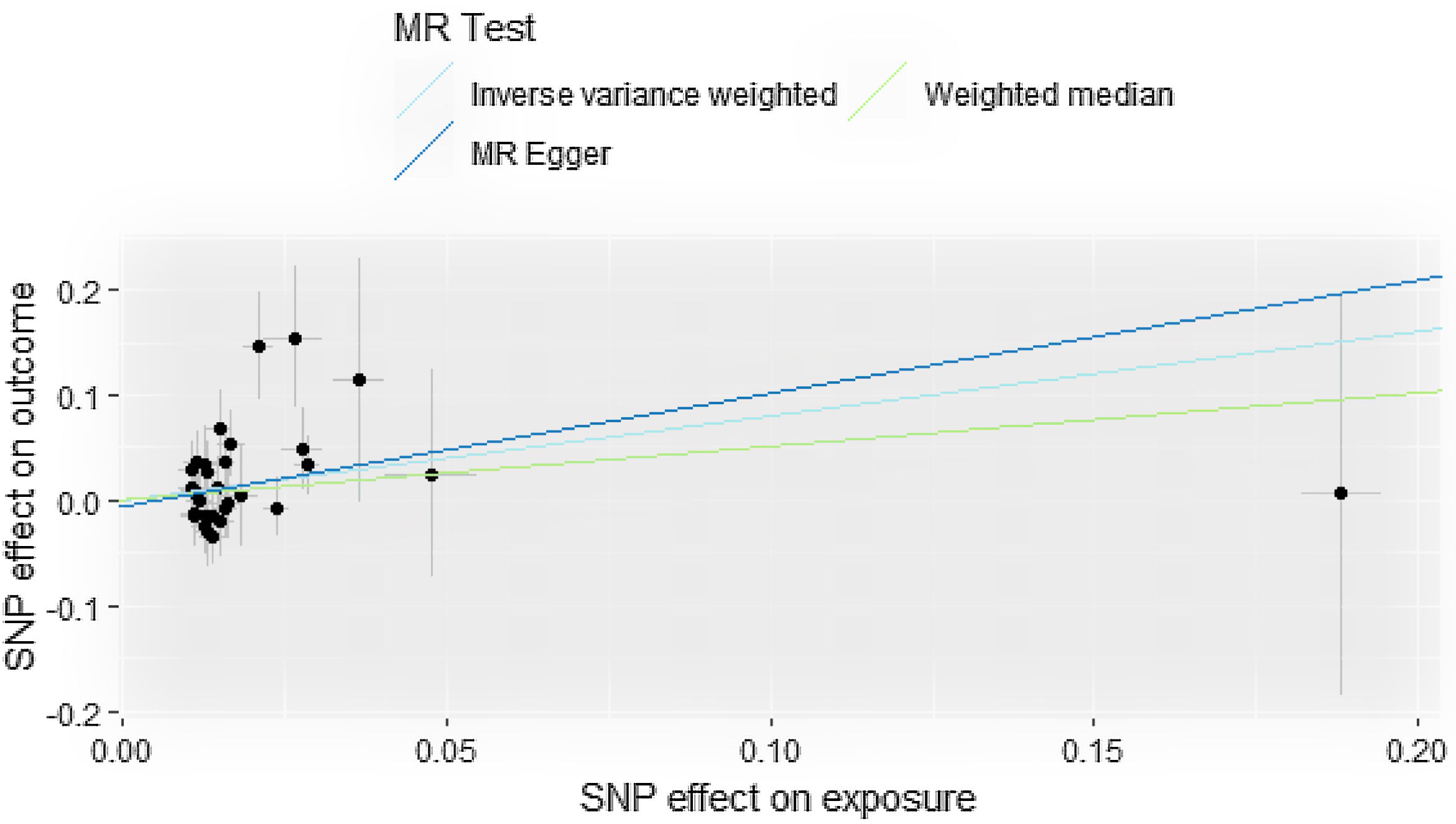
ResultsCausal effect from drinks per week to cutaneous melanomaThe pooled R for the 31 SNP associated with alcohol consumption was 0.014 and the F value was 176 (>10), indicating that the instrumental variables involved in this study were strong instrumental variables.11 Information on the final tool variables is detailed in Table 1. We found a positive and statistically significant causal effect of alcohol intake on the risk of cutaneous malignant melanoma (odds ratio [OR]: 2.23; 95% confidence interval [95%CI]: 1.11-4.47; p=0.02). Whereas our analysis using the MR Egger (OR: 2.91; 95%CI: 0.61-13.76; p=0.18) and weighted median (OR: 1.66; 95%CI: 0.63-4.36; p=0.30) methods yielded less than identical results, given the lower efficacy of both methods and the reference to previous studies in this study,12 both concluded that the results of the IVW method could prevail. We used the Cochran Q test for heterogeneity and obtained the p-value 0.51 for IVW and p-value 0.47 for MR Egger; no significant heterogeneity was observed.13 No apparently significant pleiotropy was seen (Fig. 1), and sensitivity analysis was carried out using the leave-one-out method (Fig. 2).14
Causal effect from cigarettes per day and smoking initiation to cutaneous melanomaIn the present MR study, both IVW, MR Egger, and weighted median showed no significant causal relationship between cigarettes per day and cutaneous melanoma (OR: 0.85; 95%CI: 0.54-1.35; p=0.50) or smoking initiation and cutaneous melanoma (OR: 1.02; 95%CI: 0.74-1.39; p=0.88) (Table 2). The p-values of Cochran Q test for cigarettes per day and smoking initiation were 3.583 and 0.104, respectively, and no significant heterogeneity was seen.
DiscussionThis study found that alcohol consumption showed a positive causal relationship with cutaneous malignant melanoma, whereas smoking showed no significant causal relationship with cutaneous malignant melanoma. To the best of our knowledge, this is the first original article to investigate the relationship between alcohol consumption, smoking, and other bad habits and malignant melanoma of the skin.
Previous studies have shown that when the digestive tract is directly exposed to acetaldehyde (one of the metabolites of ethanol), it greatly increases its risk of cancer, and the same result has been found in the skin.2 One possible mechanism is that when the skin is exposed to excess ethanol and acetaldehyde, it exceeds the maximum detoxification capacity of its metabolic enzymes, which in turn causes activation of the cAMP/PKA pathway and ultimately leads to the formation of macromolecular adducts.2 According to a prospective cohort study of the European Prospective Investigation into Cancer and Nutrition (EPIC), lifetime alcohol consumption is not only positively associated with melanoma of the skin (hazard ratio [HR]: 0.93; 95%CI: 0.80-1.08; p=0.13), but also with an increased risk of basal cell carcinoma.15 According to a long-term case-control study (502 melanoma patients and 565 control patients), the incidence of melanoma was significantly higher in patients who drank more than 1.4 drinks per week (OR: 1.55), with a significantly higher incidence among female drinkers than men specifically noted.16 According to Tan et al.,17 VEGF expression was significantly increased in ethanol-treated B16F10 melanoma, leading to increased angiogenesis within the tumor tissue and thus causing accelerated tumor progression. In addition, ethanol can alter the mitochondrial dynamics of tumor cells by increasing their mitochondrial division and blocking their fusion, suggesting that ethanol not only affects melanoma progression but also enhances its metastatic ability.18,19 Alcohol consumption also leads to a decrease in the number of mature B lymphocytes, NK cells, and CD8+ T cells (probably through the action of ethanol on the S1P/S1P receptor 1 pathway, which inhibits the drainage of lymphocytes from the human spleen20), which in turn accelerates the development and metastasis of melanoma.21,22 In conclusion, a positive causal relationship between alcohol consumption and the risk of cutaneous malignant melanoma has been described in a large body of literature. These findings are the same as those obtained in the present MR study. Therefore, reducing or prohibiting alcohol intake is an important step in preventing cutaneous malignant melanoma and slowing down the progression of the disease in melanoma patients.
There is extensive previous literature indicating that smoking is negatively associated with cutaneous malignant melanoma. According to a study by Jean et al., the incidence of melanoma was significantly lower in former smokers compared to never-smokers (HR: 0.76; 95%CI: 0.57-1.01), and the risk of melanoma was even lower in former smokers with higher smoking (HR: 0.75; 95%CI: 0.56-0.98 10 cigarettes/day).23 It is known that UV radiation is an important risk factor for melanoma, and it is hypothesized that the inhibitory effect of cigarettes on melanoma risk may be related to their ability to block the stimulation of melanocytes by the inflammatory response induced by UV radiation.24,25 Nicotine promotes the production of anti-inflammatory factors through cholinergic anti-inflammatory mechanisms while inhibiting the production of inflammatory factors (IL-8, IL-6, TNFA).26 On the other hand, nicotine may alter the overall condition of the skin by inhibiting the synthesis of prostacyclin, which in turn causes the constriction of peripheral blood vessels, especially in the skin.27,28 The reduction in blood flow may also inhibit the progression of melanoma to a large extent. At the same time, we should also consider that smoking is closely related to one's socioeconomic status, for example, smokers tend to have a lower socioeconomic status, and their education and health concerns are generally lower than those of nonsmokers, resulting in a significantly lower detection rate of melanoma in this population. Nevertheless, studies by Osterlind et al.29 and De Hertog et al.30 showed no significant causal relationship between smoking and cutaneous malignant melanoma. This indicates that the relationship between smoking and melanoma still needs to be further investigated. Our MR findings reveal that smoking is not a risk factor for cutaneous malignant melanoma, which is similar to the results of the above study.
There are strengths and limitations of the present study. The strengths of this study are that it avoids the bias associated with confounding factors and reverse causality that exist in observational studies, and that it does not have to face the implementation costs and ethical issues that must accompany randomized controlled trials. The present study demonstrated a causal relationship between alcohol consumption and cutaneous malignant melanoma, with no clear causal relationship between smoking and cutaneous melanoma, and our use of genetic information as a tool for causality analysis is naturally statistically strong. Nevertheless, there are some limitations in this study. The data sources of the genetic information involved in this study are European populations, so further studies are needed to find out whether the results of this study are applicable to other populations such as Asian populations. Another limitation is the presence of pleiotropy (mainly horizontal pleiotropy), which would violate the idea of Mendelian randomization if instrumental variables could directly affect the results without exposure factors. Yet we did not see any anomalies in the MR-PRESSO analysis, it also proves that our results are valid.
Overall, this study provides MR evidence supporting alcohol consumption as a risk factor for cutaneous malignant melanoma. And the causal relationship between smoking and cutaneous malignant melanoma still needs to be further investigated.
Availability of databases and material for replicationThe original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
In recent years, numerous papers have pointed out that drinking and smoking are also associated with an increased incidence of melanoma. In an article with a meta-analysis on alcohol consumption and melanoma, a moderate association between alcohol consumption and increased risk of melanoma was noted. It has also been shown that ethanol can block the developmental process of melanocytes and directly contribute to the disease progression of melanoma. As for smoking and melanoma, studies have shown that smoking is negatively associated with melanoma incidence and may even reduce its mortality rate. Nevertheless, previous studies do not exclude the influence of confounding factors and there may be bias. The causal relationship between alcohol consumption, smoking, and skin melanoma development remains undetermined.
What does this study add to the literature?We found a positive and statistically significant causal effect of alcohol intake on the risk of cutaneous malignant melanoma (OR: 2.23; 95%CI: 1.11-4.47; p=0.02). The present Mendelian randomization study showed no significant causal relationship between cigarettes per day and cutaneous melanoma (OR: 0.85; 95%CI: 0.54-1.35; p=0.50) or smoking initiation and cutaneous melanoma (OR: 1.02; 95%CI: 0.74-1.39; p=0.88).
What are the implications of the results?This study provides Mendelian randomization evidence supporting alcohol consumption as a risk factor for cutaneous malignant melanoma. And the causal relationship between smoking and cutaneous malignant melanoma still needs to be further investigated. This study used the published Genome-Wide Association Study (GWAS) pooled data on the association between alcohol consumption and smoking and genetic variation in melanoma to estimate the genetic association between exposure and disease, which has tremendous potential clinical and public health implications.
Carlos Álvarez-Dardet.
Transparency declarationThe corresponding author on behalf of the other authors guarantee the accuracy, transparency and honesty of the data and information contained in the study, that no relevant information has been omitted and that all discrepancies between authors have been adequately resolved and described.
Authorship contributionsJiaxiang Xu designed the study, performed statistical analyses and wrote the first version of the draft. Guangshuai Li revised the draft. Wenhui Liu, Xuanjun Liu and Xinlong Zhou contributed suggestions for manuscript revision. All authors read and approved the final manuscript.
AcknowledgementsOur data are from the FinnGen consortium and we thank all researchers for the collection, collation and publication of this data.
FundingNone.
Conflicts of interestNone.