To carry out a cost-utility analysis of the application of the Oncotype genomic test to inform the decision to use or not to use chemotherapy in the Basque Country (Spain).
MethodThe cost-utility study was carried out using a discrete event simulation model representing the natural history of breast cancer. The decision of treatment with chemotherapy based on Oncotype was compared with the standard of treatment based on clinical-pathological criteria. The model included clinical data from Basque hospitals and the literature and was processed by deterministic and probabilistic analysis to calculate the incremental cost-effectiveness ratio (ICER), the cost-effectiveness plane, the acceptability curve and the expected value of perfect information. The study adopted both a health and societal perspective.
ResultsFrom a health perspective, the deterministic analysis estimated an ICER for Oncotype of 17,453 euros/quality-adjusted life year (QALY), discount included, and 9,613 euros/QALY without the discount. Eighty five percent (85%) of the simulations were below the efficiency threshold for Spain. The parametric variability associated with the Oncotype results was the main uncertainty factor in the decision.
ConclusionsOncotype is a cost-effective intervention from a health system perspective since each QALY gained costs less than 25,000 euros. From a societal perspective, it is dominant since it provides greater health and is accompanied by cost savings.
Llevar a cabo un análisis de coste-utilidad del uso del test genómico Oncotype en el País Vasco (España) para informar la decisión de uso de quimioterapia.
MétodoEl estudio de coste-utilidad se realizó mediante un modelo de simulación de eventos discretos que representó la evolución natural del cáncer de mama. La decisión de tratamiento con quimioterapia basada en Oncotype se comparó con el estándar de tratamiento basado en criterios clínico-patológicos. El modelo incluyó datos clínicos de hospitales vascos y la literatura para calcular la ratio de coste-efectividad incremental (RCEI) mediante análisis determinista y probabilístico, el plano coste-efectividad, la curva de aceptabilidad y el valor esperado de la información perfecta. El estudio adoptó una perspectiva tanto sanitaria como social.
ResultadosEl análisis determinista estimó una RCEI para Oncotype de 17.453 euros/año de vida ajustado por calidad (AVAC) con descuento y 9613 euros euros/AVAC sin descuento, desde la perspectiva sanitaria. El 85% de las simulaciones estuvieron por debajo el umbral de aceptabilidad para España. La variabilidad paramétrica asociada a los resultados de Oncotype fue el mayor factor de incertidumbre de la decisión.
ConclusionesOncotype constituye una intervención coste-efectiva, ya que cada AVAC ganado tiene asociado un coste inferior a 25.000 euros. El test es dominante desde una perspectiva social al lograr mayor salud acompañada de ahorros.
The incidence of breast cancer is growing in developed countries due in part to the increase in life expectancy.1 In addition, as a diagnosis anticipating intervention, population screening has led to an increase in cases discovered in early stages.2 An important portion of these cases appear with negative lymph nodes, positive estrogen receptors and negative HER2 in which hormonal treatment has proven effective,3 but there are doubts about the risk-benefit relationship of adjuvant chemotherapy. In some cases, the adverse effects outweigh the benefit due to the low risk of recurrence and, in others, their biological characteristics are less sensitive to chemotherapy.4 In order to assist in the decision about which patients should receive chemotherapy, different clinical-pathological criteria have been developed, such as those of the National Comprehensive Cancer Network (NCCN),5 but the accuracy of their application has been suboptimal. The need for more accurate tools stimulated the development of new multigenic tests that predict the risk of metastasis and allow the selection of cases that are high risk and will therefore benefit from chemotherapy.6–8
The Oncotype test is based on the technique known as reverse transcriptase polymerase chain reaction, that analyzes five reference genes and 16 genes related to cancer and cell-associated proliferation, invasion and hormonal response.9 It was introduced into the market in 2004 and uses a continuous scale to predict the risk of recurrence of cancer at 10 years and the benefit of adjuvant chemotherapy. In 2012, the Department of Health of the Basque Government decided to incorporate it into the services portfolio of the Basque Health Service to be used in cases of early breast cancer according to the indications defined by a group of experts.10 Although Oncotype cost-effectiveness studies have been carried out, most have been developed from theoretical models based on initial indications.11 Further, its use has not been the same in all countries and a number of economic evaluations have been carried out specifically for the use of the test in each country.12,13 To fully understand the specific impact of the use of Oncotype, the cost-utility study presented here should be based on data from clinical practice in the Basque Health Service.14,15
The objective of this work was to carry out a cost-utility study of the use of the Oncotype genomic test in the Basque Health Service during the years 2012-2015 through a probabilistic model, thus addressing the corresponding gaps in the research literature and promoting better modeling of breast cancer treatment alternatives.
MethodThe research consisted of a cost-utility study based on a model representing the natural history of breast cancer. The alternative of treatment with chemotherapy according to the risk score criterion based on the Oncotype was compared with the standard alternative based on clinical-pathological criteria.16,17 The technique used was discrete events simulation with the software Arena version 14 (Rockwell Corp.). The population included and therefore simulated in the model were 401 patients with a mean age of 57 years and T1b-c/T2 localized breast cancer, positive hormonal receptors, HER2- and size N0 or N1mi participating in the clinical study.10 Data related to characteristics of patients, Oncotype score and prescribed treatment type were collected over three years of Oncotype clinical practice use in Basque hospitals.10 The treatment that would have been assigned to each patient if Oncotype had not been used, hormonal therapy alone or in combination with chemotherapy, was also recorded by oncologists before testing with Oncotype.10 Though it was not actually applied to patients, that standard alternative based on conventional clinical-pathological factors was used as comparator in the economic evaluation. The treatment recommendation after the genomic test was chemotherapy if test risk-score was high (>30), hormonotherapy alone if the risk was low (<18) and the decision took into account clinical-pathological factors if the risk was intermediate (18-30). The inclusion of Oncotype led to a decrease in the number of chemotherapies prescribed with clinical-pathological criteria, from 56% to 25% of the cases.10
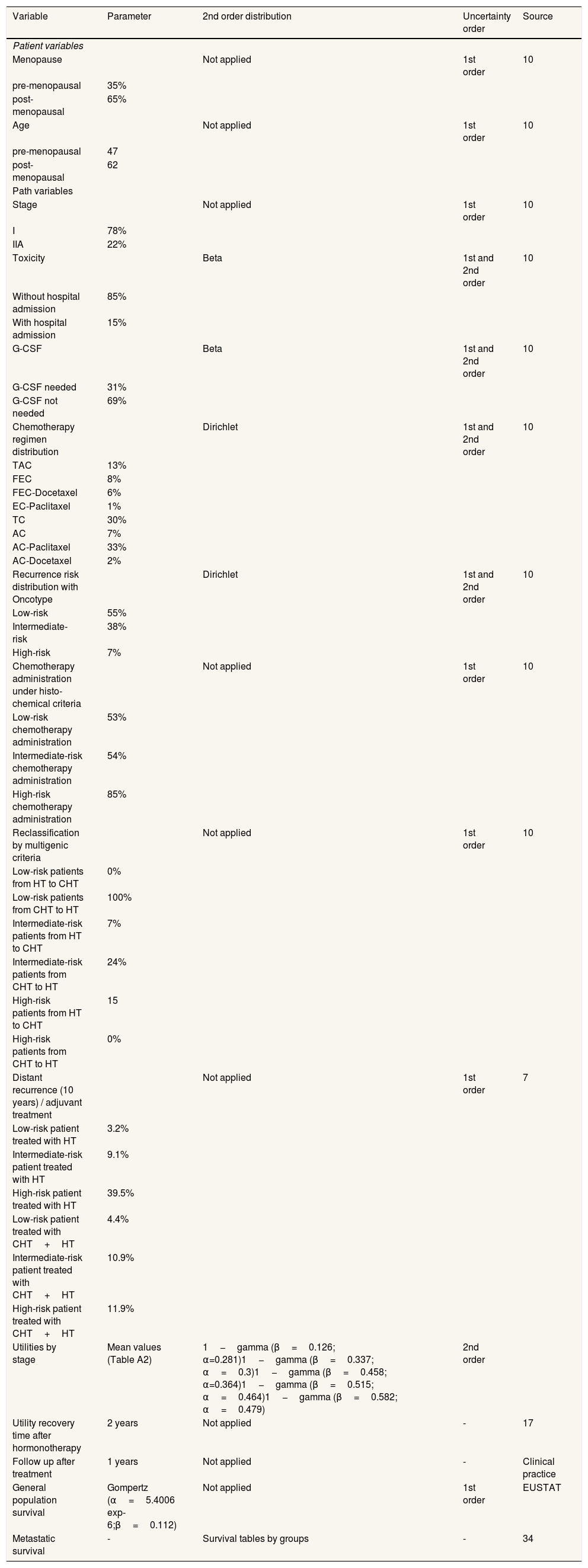
Discrete events simulation modelInstead of Markov models, we used discrete events simulation to represent the natural history of the early breast cancer because of its capacity to take into account patient history in the progression through the model.18 The discrete events simulation model developed for the study reproduced two identical populations of patients whose pathways were different according to the criteria applied for the prescription of chemotherapy. In turn, the simulated populations constituted representations of the patients included in the sample of participating hospitals, in order to simulate the population on which real data were available (Table 1 and Figure A3 in the online Appendix).. The follow-up period with the populations started from the moment they were evaluated in order to make a decision about the adjuvant treatment until the end of their lives, either due to death derived from a recurrence or due to causes other than breast cancer. Figure A1 in the online Appendix shows the flow diagram of the natural history of hormone receptor positive/HER2 negative early breast cancer. The path that each patient followed depended on her initial characteristics (age, stage and menopause status) and by the risk group and criteria applied (oncotype or clinical-pathological) which determined her probability of experiencing recurrence. Patients’ initial characteristics (age, stage and menopause status) were assigned by jointly sampling from the patient-level dataset to preserve the correlations among them. A discount of 3% per annum was applied both for costs and for utility measured in quality adjusted life years (QALY).19 In a complementary way, a calculation was carried out in which no discount was applied. The technical report that describes the model thoroughly has been included in the online Appendix.
Model parameters for the representation of the natural history of breast cancer.
| Variable | Parameter | 2nd order distribution | Uncertainty order | Source |
|---|---|---|---|---|
| Patient variables | ||||
| Menopause | Not applied | 1st order | 10 | |
| pre-menopausal | 35% | |||
| post-menopausal | 65% | |||
| Age | Not applied | 1st order | 10 | |
| pre-menopausal | 47 | |||
| post-menopausal | 62 | |||
| Path variables | ||||
| Stage | Not applied | 1st order | 10 | |
| I | 78% | |||
| IIA | 22% | |||
| Toxicity | Beta | 1st and 2nd order | 10 | |
| Without hospital admission | 85% | |||
| With hospital admission | 15% | |||
| G-CSF | Beta | 1st and 2nd order | 10 | |
| G-CSF needed | 31% | |||
| G-CSF not needed | 69% | |||
| Chemotherapy regimen distribution | Dirichlet | 1st and 2nd order | 10 | |
| TAC | 13% | |||
| FEC | 8% | |||
| FEC-Docetaxel | 6% | |||
| EC-Paclitaxel | 1% | |||
| TC | 30% | |||
| AC | 7% | |||
| AC-Paclitaxel | 33% | |||
| AC-Docetaxel | 2% | |||
| Recurrence risk distribution with Oncotype | Dirichlet | 1st and 2nd order | 10 | |
| Low-risk | 55% | |||
| Intermediate-risk | 38% | |||
| High-risk | 7% | |||
| Chemotherapy administration under histo-chemical criteria | Not applied | 1st order | 10 | |
| Low-risk chemotherapy administration | 53% | |||
| Intermediate-risk chemotherapy administration | 54% | |||
| High-risk chemotherapy administration | 85% | |||
| Reclassification by multigenic criteria | Not applied | 1st order | 10 | |
| Low-risk patients from HT to CHT | 0% | |||
| Low-risk patients from CHT to HT | 100% | |||
| Intermediate-risk patients from HT to CHT | 7% | |||
| Intermediate-risk patients from CHT to HT | 24% | |||
| High-risk patients from HT to CHT | 15 | |||
| High-risk patients from CHT to HT | 0% | |||
| Distant recurrence (10 years) / adjuvant treatment | Not applied | 1st order | 7 | |
| Low-risk patient treated with HT | 3.2% | |||
| Intermediate-risk patient treated with HT | 9.1% | |||
| High-risk patient treated with HT | 39.5% | |||
| Low-risk patient treated with CHT+HT | 4.4% | |||
| Intermediate-risk patient treated with CHT+HT | 10.9% | |||
| High-risk patient treated with CHT+HT | 11.9% | |||
| Utilities by stage | Mean values (Table A2) | 1−gamma (β=0.126; α=0.281)1−gamma (β=0.337; α=0.3)1−gamma (β=0.458; α=0.364)1−gamma (β=0.515; α=0.464)1−gamma (β=0.582; α=0.479) | 2nd order | |
| Utility recovery time after hormonotherapy | 2 years | Not applied | - | 17 |
| Follow up after treatment | 1 years | Not applied | - | Clinical practice |
| General population survival | Gompertz (α=5.4006 exp-6;β=0.112) | Not applied | 1st order | EUSTAT |
| Metastatic survival | - | Survival tables by groups | - | 34 |
G-CSF: granulocyte colony-stimulating factor; HT: hormonotherapy; CHT: chemotherapy; TAC: docetaxel + doxorubicina + ciclofosfamida; FEC: fluorouracilo + epirubicina + ciclofosfamida; EC: epirubicina + ciclofosfamida; TC: docetaxel + ciclofosfamida; AC: doxorubicina + ciclofosfamida.
The utilities of the different states in relation to the breast cancer (Table A2 in the online Appendix) were estimated following the work of Stout et al.20 based on the loss of utility caused by the treatment according to each age group and health status. For that, we used data from the 2011-2012 Spanish National Health Survey, which contains the EQ-5D-5L instrument.21,22
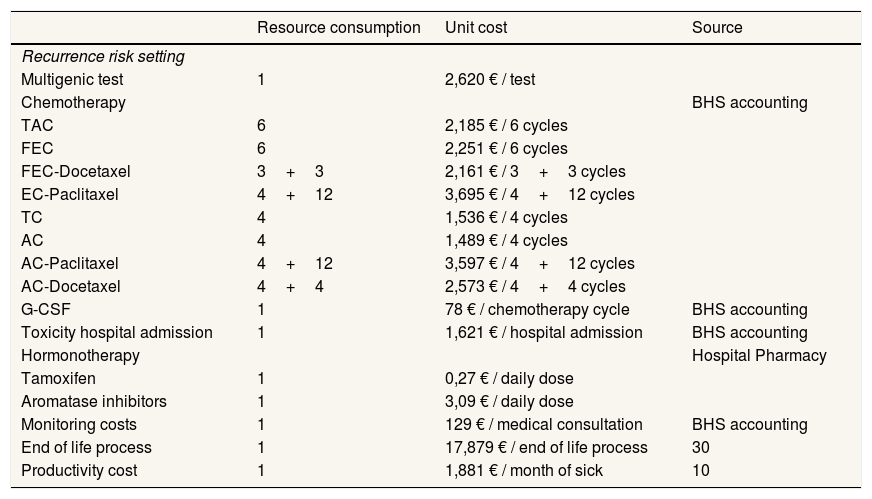
Cost analysisThe baseline case assumed the perspective of the health system, incorporating only health-care costs, which were calculated by multiplying the use of resources by each unit cost (Table 2).10 An analysis was then added from the societal perspective in which costs for loss of productivity due to sick leave due to chemotherapy were included.10,14,19
Unit costs of the resources included in the model.
| Resource consumption | Unit cost | Source | |
|---|---|---|---|
| Recurrence risk setting | |||
| Multigenic test | 1 | 2,620 € / test | |
| Chemotherapy | BHS accounting | ||
| TAC | 6 | 2,185 € / 6 cycles | |
| FEC | 6 | 2,251 € / 6 cycles | |
| FEC-Docetaxel | 3+3 | 2,161 € / 3+3 cycles | |
| EC-Paclitaxel | 4+12 | 3,695 € / 4+12 cycles | |
| TC | 4 | 1,536 € / 4 cycles | |
| AC | 4 | 1,489 € / 4 cycles | |
| AC-Paclitaxel | 4+12 | 3,597 € / 4+12 cycles | |
| AC-Docetaxel | 4+4 | 2,573 € / 4+4 cycles | |
| G-CSF | 1 | 78 € / chemotherapy cycle | BHS accounting |
| Toxicity hospital admission | 1 | 1,621 € / hospital admission | BHS accounting |
| Hormonotherapy | Hospital Pharmacy | ||
| Tamoxifen | 1 | 0,27 € / daily dose | |
| Aromatase inhibitors | 1 | 3,09 € / daily dose | |
| Monitoring costs | 1 | 129 € / medical consultation | BHS accounting |
| End of life process | 1 | 17,879 € / end of life process | 30 |
| Productivity cost | 1 | 1,881 € / month of sick | 10 |
BHS: Basque Public Health Service; G-CSF: granulocyte colony-stimulating factor; TAC: docetaxel + doxorubicina + ciclofosfamida; FEC: fluorouracilo + epirubicina + ciclofosfamida; EC: epirubicina + ciclofosfamida; TC: docetaxel + ciclofosfamida; AC: doxorubicina + ciclofosfamida.
First, the calculation of the incremental cost-effectiveness ratio (ICER), composed of incremental cost and incremental effectiveness, was carried out through a deterministic analysis using the mean of each parameter. Subsequently, a probabilistic sensitivity analysis was developed with 1000 simulations in which the applied values varied in each replication according to the distributions used (Table A1 in the online Appendix).16 The result of each trial is summarized in an ICER used to obtain the confidence interval (CI) of the mean ICER through bootstrap,23,24 as well as the CI of the total cloud of ICERs (percentiles 2.5 and 97.5), the cost-effectiveness plane and the acceptability curves. Given parameter uncertainty, in some simulations the preferred strategy would have been the rejected one and, thus, a negative net benefit would have occurred. The average opportunity loss of all simulations was calculated later, and represented the expected cost of the uncertainty, which is equivalent to the total expected value of perfect information (EVPI) per patient. This estimate is multiplied by the target population of the technology for the next five years (1505 patients according to the Basque Cancer Registry) to obtain the population EVPI.16,25 To calculate the partial EVPI (EVPPI), two groups of parameters were analyzed (risk score distribution, and adverse effects probabilities).16,25
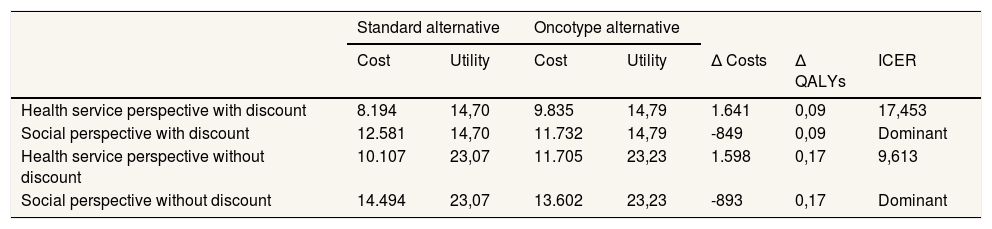
ResultsThe deterministic cost-utility analysis from the health system perspective estimated an ICER of 17,453 euros/QALY with the discount and 9,613 euros/QALY without the discount (Table 3). The result from the perspective of society as a whole is shown in the same table. Thus, when productivity costs were included, the evaluated intervention was dominant since it determined cost savings and health gains.
Results of the deterministic cost-utility analysis comparing the clinical-pathological alternative with the Oncotype genomic test alternative.
| Standard alternative | Oncotype alternative | ||||||
|---|---|---|---|---|---|---|---|
| Cost | Utility | Cost | Utility | Δ Costs | Δ QALYs | ICER | |
| Health service perspective with discount | 8.194 | 14,70 | 9.835 | 14,79 | 1.641 | 0,09 | 17,453 |
| Social perspective with discount | 12.581 | 14,70 | 11.732 | 14,79 | -849 | 0,09 | Dominant |
| Health service perspective without discount | 10.107 | 23,07 | 11.705 | 23,23 | 1.598 | 0,17 | 9,613 |
| Social perspective without discount | 14.494 | 23,07 | 13.602 | 23,23 | -893 | 0,17 | Dominant |
ICER: incremental cost-effectiveness ratio; QALY: quality adjusted life year.
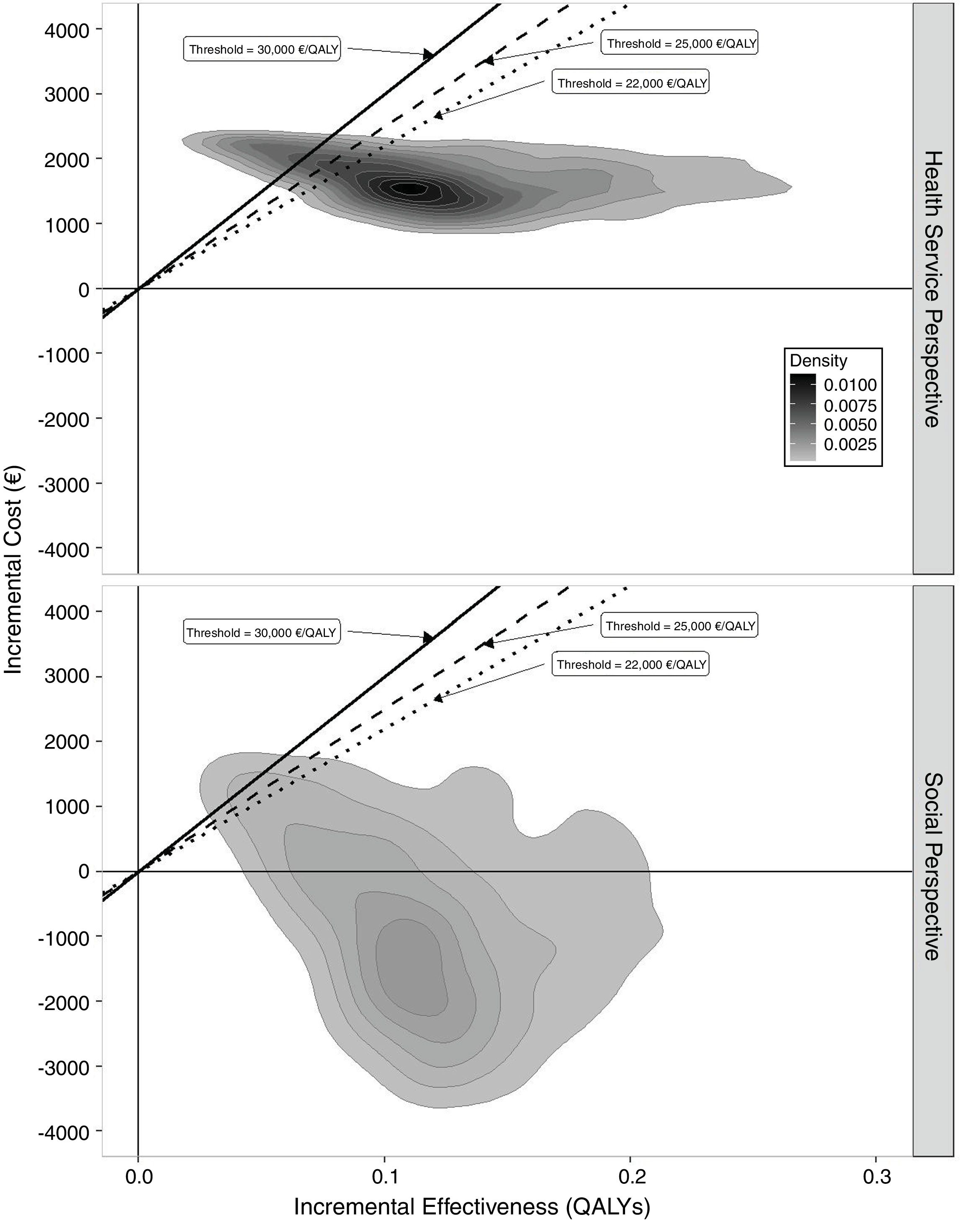
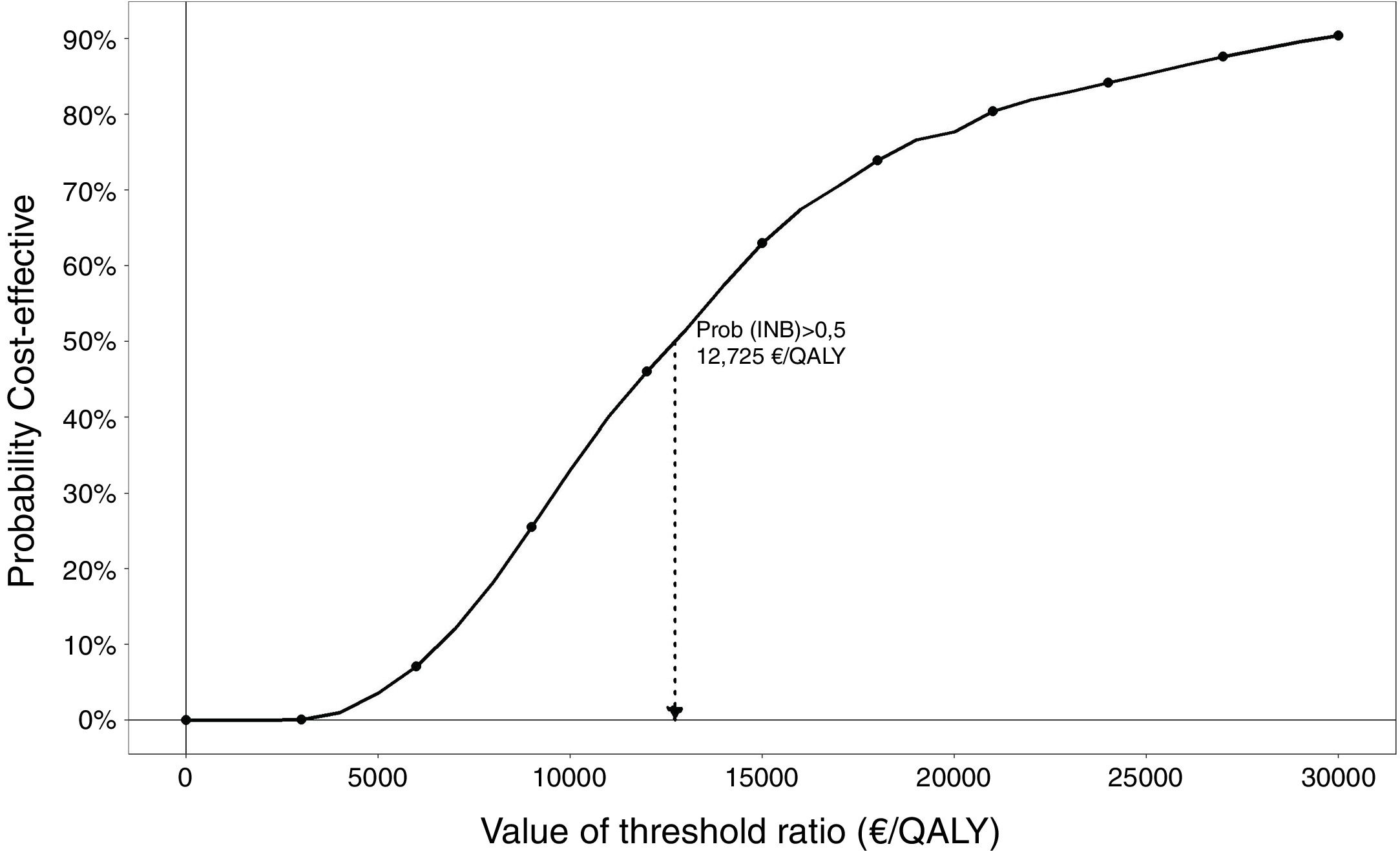
The results of the probabilistic analysis are shown in Figures 1, 2 and 3. The 95%CI for the mean ICER was (15,371€/QALY – 16,909€/QALY) and the CI for the total ICERs cloud was (4,722€/QALY – 51,345€/QALY). In the cost-effectiveness plane (Fig. 1) the results of all the simulations appear in the northeast quadrant of the cost-effectiveness plane from the perspective of the Basque Health Service with discount that is characterized by positive incremental costs and utilities; efficiency relies on the adopted threshold. Applying the societal perspective, the results appear in both northeast and southeast quadrants. The cost-effectiveness plane obtained without discount from health service and societal perspectives are shown in Figure A2 in the online Appendix. The acceptability curve (Fig. 2) displays the percentage of simulations in which the ICER is below each threshold of efficiency or willingness to pay from the perspective of the Basque Health Service and with the discount. The percentage of simulations lower than the threshold of 25,000 euros/QALY is 85%. The figure is higher still when the discount is not applied.
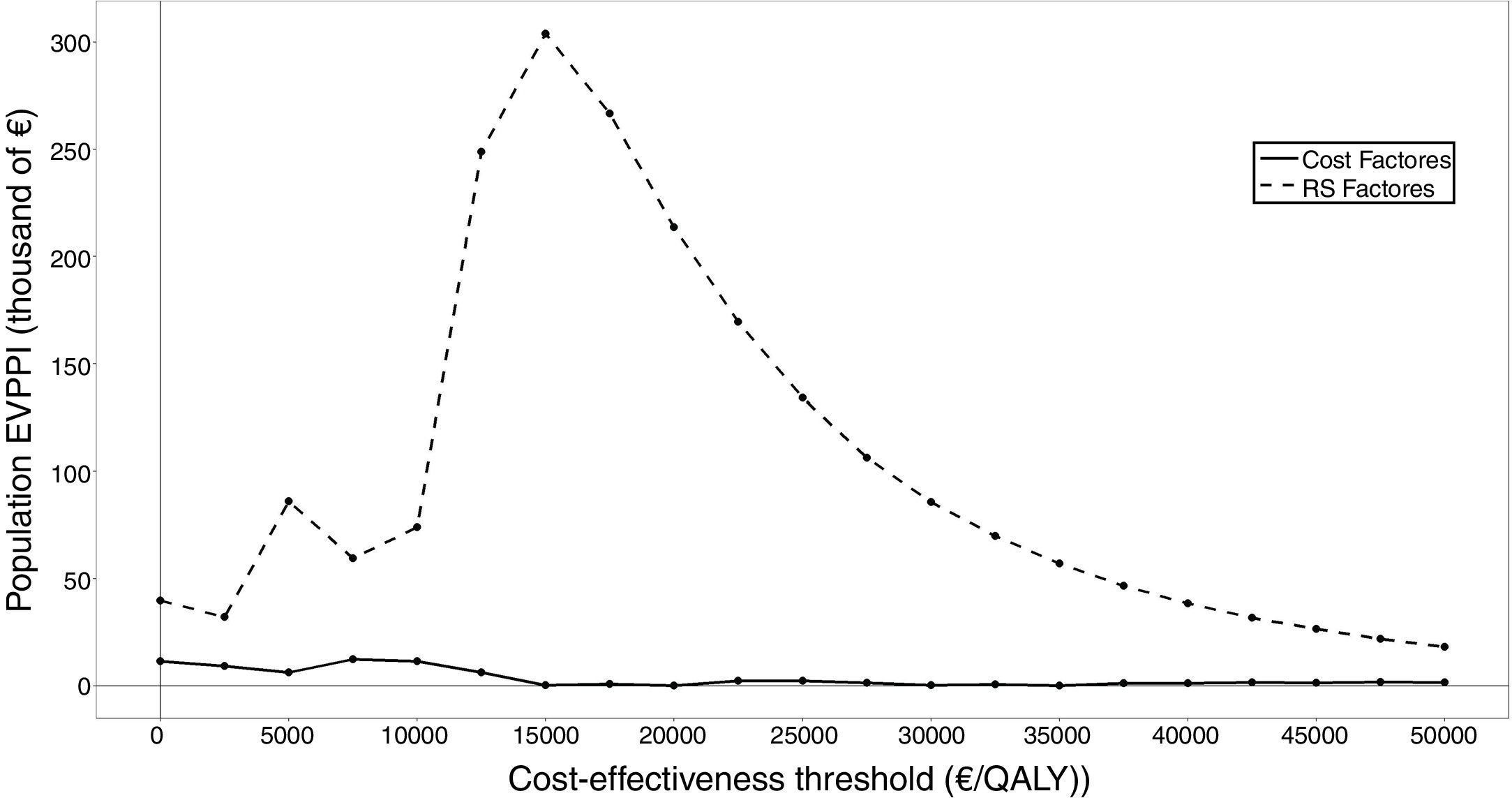
When all the parameters were varied, the total EVPI for the target population was 86,333 euros. The EVPPI synthesized in Figure 3 allowed identifying treatment decision (risk score and chemotherapy distribution) as the group of parameters that provided the greatest variability to the final result not only for the threshold of 25,000 euros/QALY estimated for the Spanish national health system26 but for the entire range of thresholds included in the calculation. The adverse effects hardly generated risk of being wrong with the decision to use Oncotype.
DiscussionThe use of the Oncotype genomic test in the Basque Health Service is a cost-effective intervention since each QALY gained costs less than 25,000 euros. If sick leave costs due to chemotherapy are included in the analysis, the result is a net saving. Thus, from the societal perspective, the Oncotype option is dominant since it generates positive health results and is accompanied by savings in the use of resources.
This decision model has two key characteristics. The first is the precision with which the genomic test establishes the risk of recurrence since the clinical-pathological criteria have important weaknesses. An example is the size of the tumor that is included in these criteria but which presented a non-significant ROC curve in the clinical study.10 In general, clinical-pathological criteria approach decision-making conservatively, prescribing chemotherapy in doubtful cases. The result is unsatisfactory because the percentage of clear cases is small. The number of chemotherapies can be reduced with the test since the improvement of the predictive values allows for a significant reduction in the group of doubtful patients. Thus, patients gain quality of life because a substantial percentage of chemotherapies can be avoided. The reduction of chemotherapies by one third of the total sample is in line with other studies.27 The second key characteristic concerns the cost of chemotherapy. The uncertainty associated with the parameters of the model indicates that in only 85% of the simulations is the ICER lower than the efficiency threshold. The probabilistic analysis allows for identification of groups of parameters that generate uncertainty in treatment decisions. The EVPPI estimation demonstrated that the parametric uncertainty about the result of the test was a relevant issue given the sample size of the clinical study.10 In each simulation, taking a set of probabilities from the two Dirichlet distributions that represented the probability of the score being in a particular group (<18, 18-30,>30) and the cost of chemotherapies, determined a loss of opportunity for the decision to apply Oncotype.
This result is consistent with other studies in which the use of Oncotype is dominant from a health perspective.11,13 Compared with our unit costs, the main difference with respect to other research is in the figure for chemotherapy cost, which is significantly higher in the literature. Thus, Smyth et al.13 applied a total cost of 7,903 euros in 2014 for each patient in Ireland treated with chemotherapy. This cost included pharmacy costs (2,161 euros) and bone marrow stimulators such as granulocyte colony-stimulating factor with pegfilgrastim (4985 euros)13. By contrast, the unit cost of chemotherapy in the Basque Health Service is much lower (2400-2500 euros).10 It is clearly the case that the higher the cost of the chemotherapy, the higher the efficiency of a genomic test that avoids or diminishes it and our probabilistic analysis quantitatively supports this relationship.
Other economic evaluations carried out in France, Mexico and the Netherlands concluded that the use of Oncotype is cost-effective from the Health Service perspective28–30 and dominant from the societal perspective when incorporating lost productivity costs.30 The value of information analysis of Oncotype was addressed in the study by Hall et al.31 to inform the planning of a clinical trial in the United Kingdom. They estimated that Oncotype is dominant but with big confidence intervals, justifying a high total EVPI. We cannot compare their results with ours because they apply Oncotype to patients with higher risk of recurrence. For them, the standard alternative was not clinical-pathological criteria, but prescribing chemotherapy directly. Our conceptual models are similar to those developed in different countries as they are structured on the recurrence risk according to different criteria and treatments. Our results are also consistent with a systematic review of the economic evaluation of genomic tests for early breast cancer care which estimates that genomic testing results in a moderate increase in total costs, but with an ICER below the threshold.32 Another systematic review points out the risk of bias in some of the economic evaluations because of source of the funding.33 This limitation does not affect our study.
From the methodological point of view, we should note a weakness in our study due to selection bias derived from the observational design. But target populations for Oncotype have been tailored to each country and our model had, necessarily, to be tied to the features of Basque clinical practice that were different from others.10,31 Another limitation to be mentioned in our analysis of the EVPI concerns the uncertainty about the use of chemotherapy that is not purely random in statistical terms but reflects differences in clinical practice. Despite these, we have included the distribution of the different treatment schemes because it highlights the added value of probabilistic processing and its key role in the estimation of the efficiency of Oncotype. At the same time, it allows to show that if the cost of oncological medicines increases, the efficiency of the use of the Oncotype will improve. Using a threshold amount the system would be willing to pay in the economic evaluations carried out in the Spanish health sector is, for the time being, purely academic, since the health authorities have not defined a reference figure or a methodology to support it. Traditionally the threshold of 30,000 euros/QALY proposed by Sacristán et al.34 in 2002 from a review of the literature has been used. Recently, Vallejo-Torres et al.26 have proposed a figure between 22,000 and 25,000 euros. The incremental cost of Oncotype Dx is found to be lower than the maximum per QALY suggested as appropriate for Spain, although, at this time, Spanish policy makers do not formally support a fixed definition for a threshold of acceptability.
In conclusion, this case of personalized medicine to inform decision-making in early breast cancer treatment resulted in a cost-effective use of resources in the Basque Health Service according to the results of the cost-utility analysis.
The clinical-pathological criteria used to decide which breast cancer patients in early stages should receive chemotherapy are not precise. The Oncotype genomic test has documented a high diagnostic validity. In 2012, the Basque Health Service decided to incorporate it into the service portfolio.
What does this study add to the literature?The use of Oncotype in the Basque Health Service is an efficient intervention since each gained quality-adjusted life year costs less than 25,000 euros, which is dominant when including productivity loss costs.
David Epstein.
Transparency declarationThe corresponding author on behalf of the other authors guarantee the accuracy, transparency and honesty of the data and information contained in the study, that no relevant information has been omitted and that all discrepancies between authors have been adequately resolved and described.
Authorship contributionsO. Ibarrondo has participated in the design of the study, the construction and processing of the model and the writing of the article. A. Arrospide has participated in the design of the study, the construction and processing of the model and has reviewed the manuscript. I. Álvarez-López, E. Galve-Calvo, M. Gutiérrez-Toribio and A. Plazaola have participated in the design of the study, the field work and the interpretation of the results. J. Mar and F. Freundlich have contributed to the design of the study, collaborated in the interpretation of results and reviewed the article, providing valuable suggestions.
AcknowledgmentsThe authors thank Purificación Martínez del Prado, Nerea Ancizar-Lizarraga, Severina Domínguez, Ainhara Lahuerta, Patricia Novas-Vidal, Cristina Churruca, Garbiñe Unanue and Lide Larburu for their contribution to the collection and assembly of data.
FundingThis work has been carried out within the doctoral thesis project of O. Ibarrondo and has not received external funding.
Conflicts of interestsNone.